Question: 6. Lil is found to have the same structure as NaCl. Given this information, estimate the lattice energy for Lil using the Born-Mayer equation with
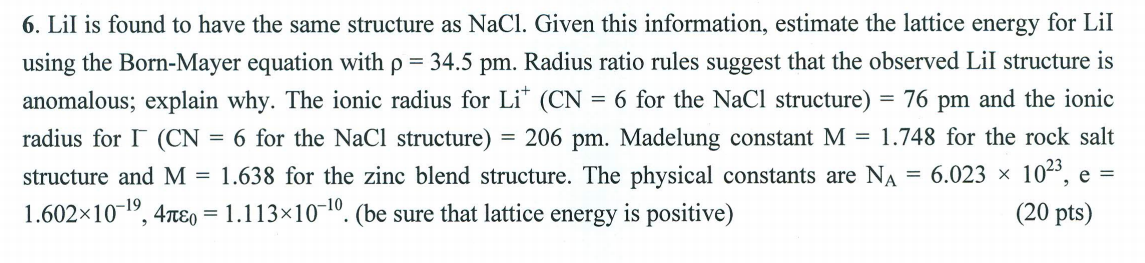
6. Lil is found to have the same structure as NaCl. Given this information, estimate the lattice energy for Lil using the Born-Mayer equation with p = 34.5 pm. Radius ratio rules suggest that the observed Lil structure is anomalous; explain why. The ionic radius for Lit (CN = 6 for the NaCl structure) = 76 pm and the ionic radius for I (CN = 6 for the NaCl structure) = 206 pm. Madelung constant M = 1.748 for the rock salt structure and M = 1.638 for the zinc blend structure. The physical constants are Na = 6.023 * 1023, e = 1.602x10-19, 4 1.113x10-10.(be sure that lattice energy is positive) (20 pts) = = 9 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


