Question: 6. Work through Example 14-10A from the text (not homework section), and complete the following items: a. Complete the stoichiometric table b. Calculate the equilibrium
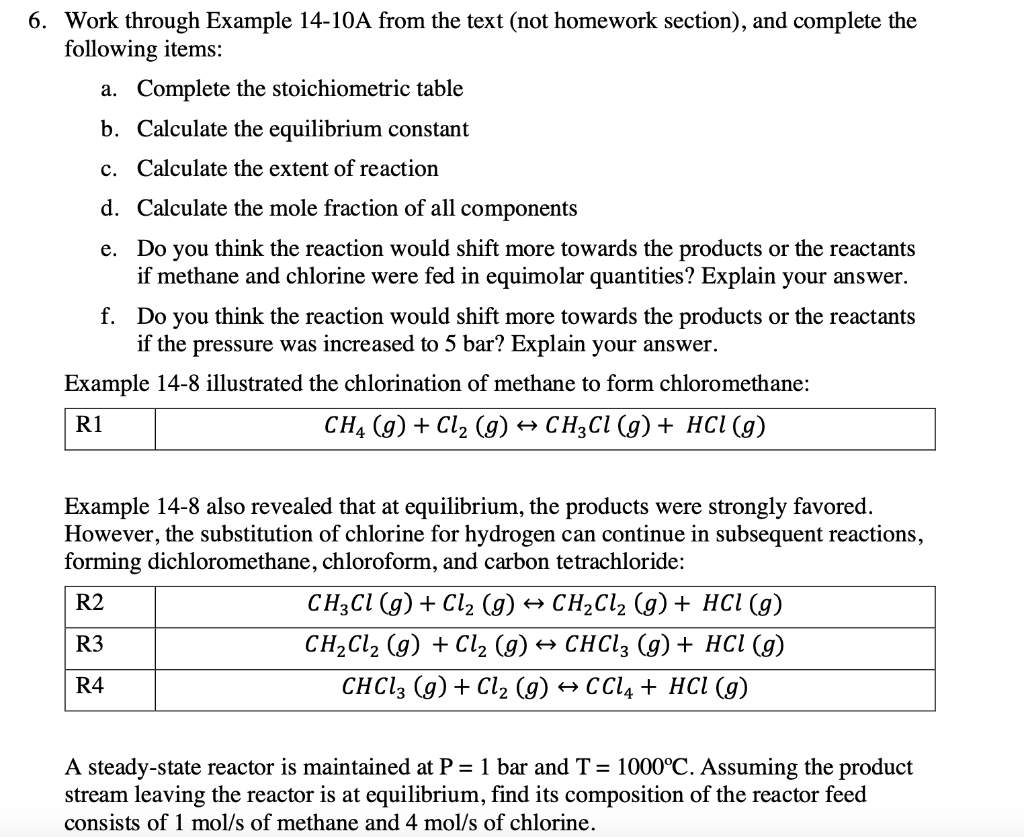
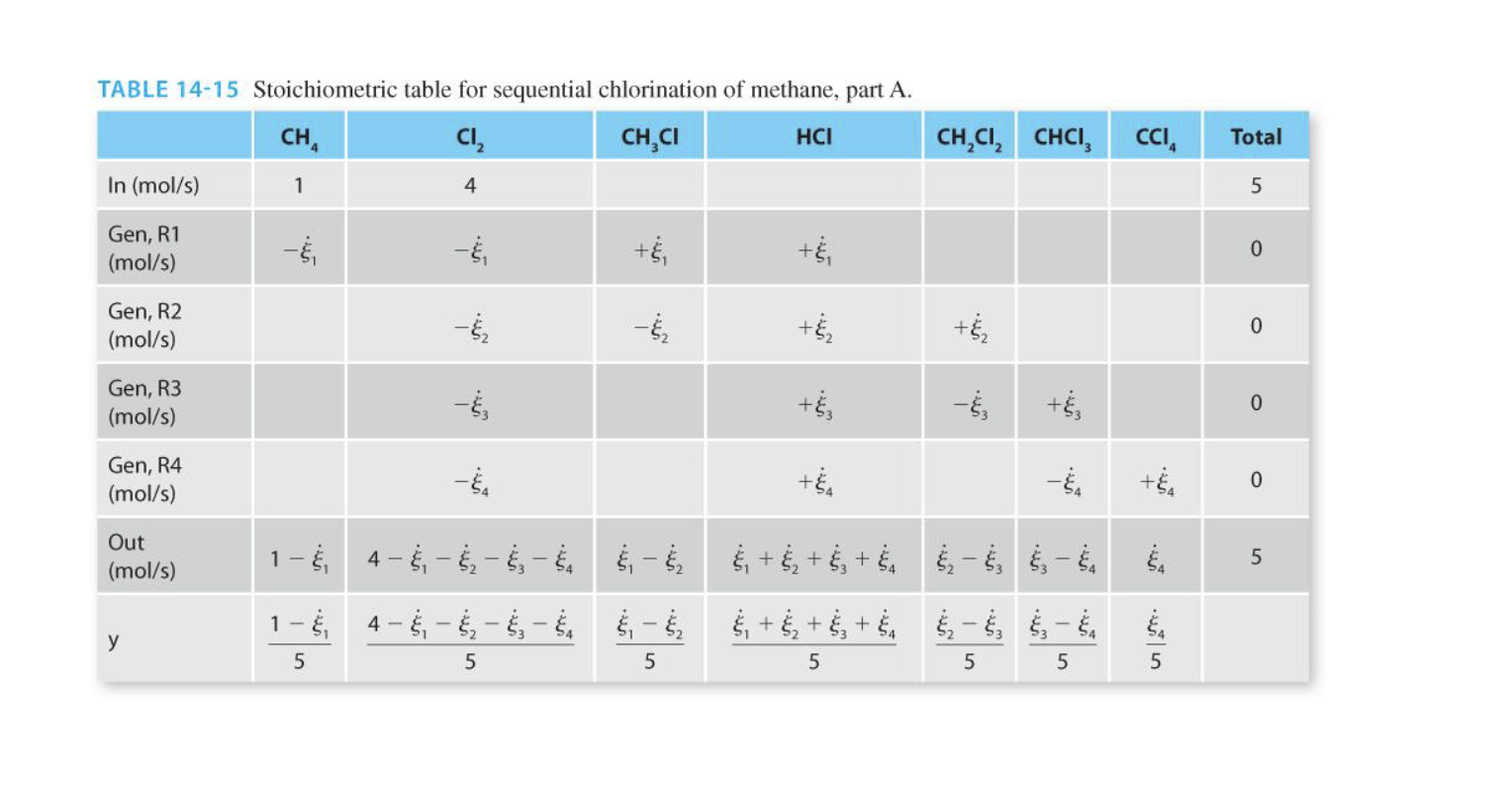
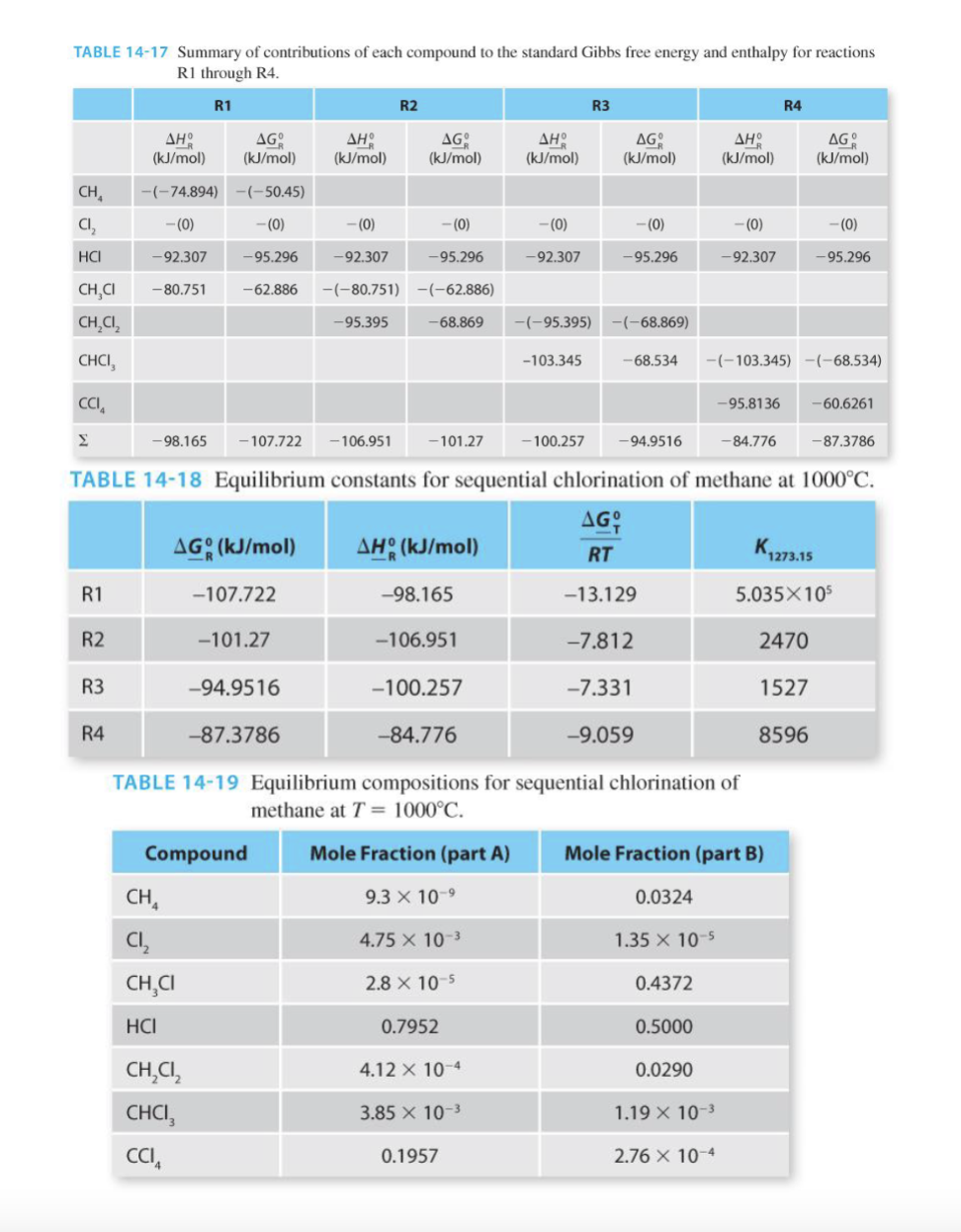
6. Work through Example 14-10A from the text (not homework section), and complete the following items: a. Complete the stoichiometric table b. Calculate the equilibrium constant c. Calculate the extent of reaction d. Calculate the mole fraction of all components e. Do you think the reaction would shift more towards the products or the reactants if methane and chlorine were fed in equimolar quantities? Explain your answer. f. Do you think the reaction would shift more towards the products or the reactants if the pressure was increased to 5 bar? Explain your answer. Example 14-8 illustrated the chlorination of methane to form chloromethane: R1 CH4 (9) + Cl2(g) + CH3Cl (g) + HCl(g) Example 14-8 also revealed that at equilibrium, the products were strongly favored. However, the substitution of chlorine for hydrogen can continue in subsequent reactions, forming dichloromethane, chloroform, and carbon tetrachloride: R2 CH3Cl (g) + Cl2 (g) + CH2Cl2 (g) + HCl (9) R3 CH2Cl2 (g) + Cl2 (g) + CHCl3 (g) + HCl (9) CHCl3 (g) + Cl2 (9) + CCl4 + HCl (g) R4 A steady-state reactor is maintained at P = 1 bar and T = 1000C. Assuming the product stream leaving the reactor is at equilibrium, find its composition of the reactor feed consists of 1 mol/s of methane and 4 mol/s of chlorine. TABLE 14-15 Stoichiometric table for sequential chlorination of methane, part A. CH, CI, CH,C HCI In (mol/s) 1 CH,C, CHCI CCI, Total 4 5 Gen, R1 (mol/s) + o Gen, R2 (mol/s) -5, - + +j 0 Gen, R3 (mol/s) - +, -, 0 . Gen, R4 (mol/s) + + 0 . Out (mol/s) 4 5 1- & 4 - , & & + 8 + 8 + 86 - , , - , 1 - $ 4- &; ; ; - ; &, + + $; + $ - $, - g | 5 5 5 5 5 5 5 TABLE 14-17 Summary of contributions of each compound to the standard Gibbs free energy and enthalpy for reactions R1 through R4. R1 R2 R3 R4 (kJ/mol) AG (kJ/mol) AH (kJ/mol) AG (kJ/mol) (kJ/mol) AG (kJ/mol) (kJ/mol) AG (kJ/mol) CH -(-74.894) -(-50.45) CI -(0) -(0) ---(0) -(0) -(0) ---(0) --(0) -(0) HCI -92.307 -95.296 -92.307 -95.296 -92.307 -95.296 -92.307 -95.296 -80.751 -62.886 -(-80.751) -(-62.886) CHCI CH,C, -95.395 -68.869 -(-95.395) -(-68.869) CHCI, -103.345 68.534 -(-103.345) -(-68.534) CCIA -95.8136 -60.6261 -98.165 - 107.722 -106.951 -101.27 -100.257 -94.9516 - 84.776 -87.3786 TABLE 14-18 Equilibrium constants for sequential chlorination of methane at 1000C. AG; AGO (kJ/mol) AH: (kJ/mol) RT 1273.15 R1 -107.722 -98.165 -13.129 5.035X 10 R2 -101.27 -106.951 -7.812 2470 R3 -94.9516 -100.257 -7.331 1527 R4 -87.3786 -84.776 -9.059 8596 TABLE 14-19 Equilibrium compositions for sequential chlorination of methane at T = 1000C. Compound Mole Fraction (part A) Mole Fraction (part B) CH 9.3 X 10-9 0.0324 4.75 X 10-3 1.35 x 10-5 CI, CHCI 2.8 x 10-5 0.4372 HCI 0.7952 0.5000 4.12 x 10-4 0.0290 CH,C1, CHCI CCI 3.85 X 10-3 1.19 X 10-3 0.1957 2.76 X 10-4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


