Question: 6. Write the oxidation numbers for the underlined in the following molecules, ions, and compounds: 18 points a 10.1 b. Sn c. Cr(OH)3 d.
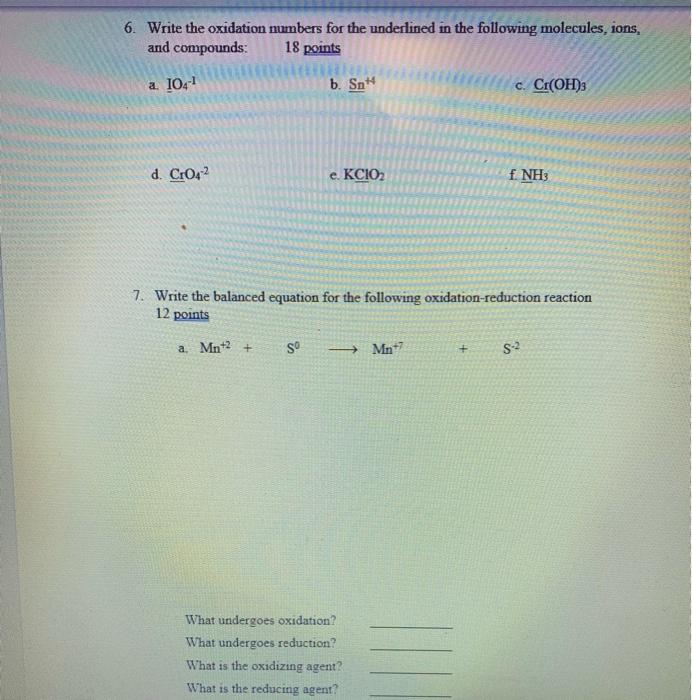
6. Write the oxidation numbers for the underlined in the following molecules, ions, and compounds: 18 points a 10.1 b. Sn c. Cr(OH)3 d. CrO42 e. KCIO2 f NH3 7. Write the balanced equation for the following oxidation-reduction reaction 12 points a. Mn*2 + Mn*7 What undergoes oxidation? What undergoes reduction? What is the oxidizing agent? What is the reducing agent?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


