Question: 7. Each individual atomic nucleus in a molecule has a characteristic chemical shift, and therefore the number of signals in an NMR spectrum is correlated
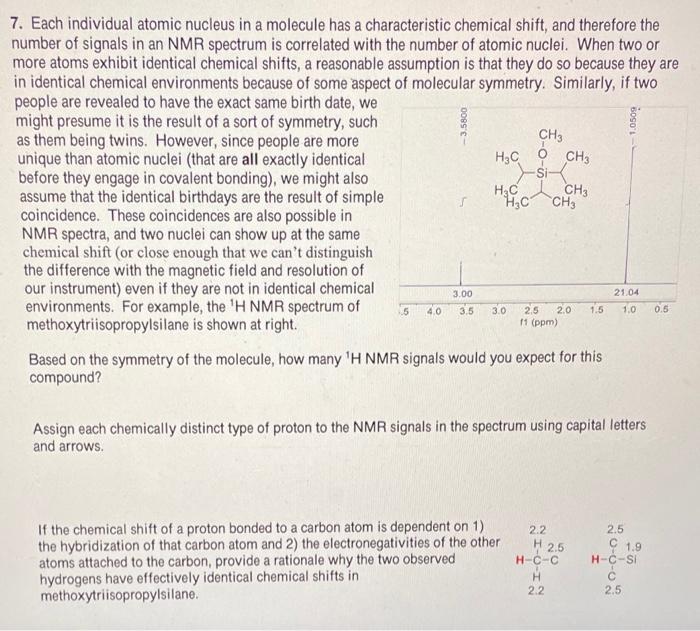
7. Each individual atomic nucleus in a molecule has a characteristic chemical shift, and therefore the number of signals in an NMR spectrum is correlated with the number of atomic nuclei. When two or more atoms exhibit identical chemical shifts, a reasonable assumption is that they do so because they are in identical chemical environments because of some aspect of molecular symmetry. Similarly, if two people are revealed to have the exact same birth date, we might presume it is the result of a sort of symmetry, such as them being twins. However, since people are more unique than atomic nuclei (that are all exactly identical before they engage in covalent bonding), we might also assume that the identical birthdays are the result of simple coincidence. These coincidences are also possible in NMR spectra, and two nuclei can show up at the same chemical shift (or close enough that we can't distinguish the difference with the magnetic field and resolution of our instrument) even if they are not in identical chemical environments. For example, the 1H NMR spectrum of methoxytriisopropylsilane is shown at right. Based on the symmetry of the molecule, how many 1H NMR signals would you expect for this compound? Assign each chemically distinct type of proton to the NMR signals in the spectrum using capital letters and arrows. If the chemical shift of a proton bonded to a carbon atom is dependent on 1 ) the hybridization of that carbon atom and 2) the electronegativities of the other atoms attached to the carbon, provide a rationale why the two observed hydrogens have effectively identical chemical shifts in methoxytriisopropylsilane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


