Question: 7. In a simple classical model for hydrogen, it can have a dipole moment of about 110-23 Am. Assuming the radius of hydrogen is
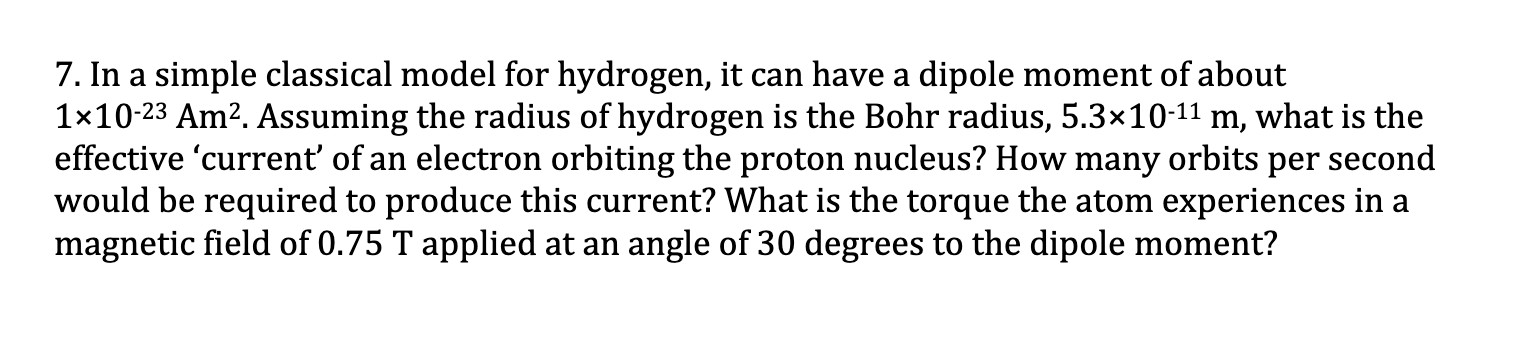
7. In a simple classical model for hydrogen, it can have a dipole moment of about 110-23 Am. Assuming the radius of hydrogen is the Bohr radius, 5.310-11 m, what is the effective 'current' of an electron orbiting the proton nucleus? How many orbits per second would be required to produce this current? What is the torque the atom experiences in a magnetic field of 0.75 T applied at an angle of 30 degrees to the dipole moment?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


