Question: 7. Use the table below to answer the questions that follow. a. Which of the solutions has the highest osmolarity? (1 point) b. Which of
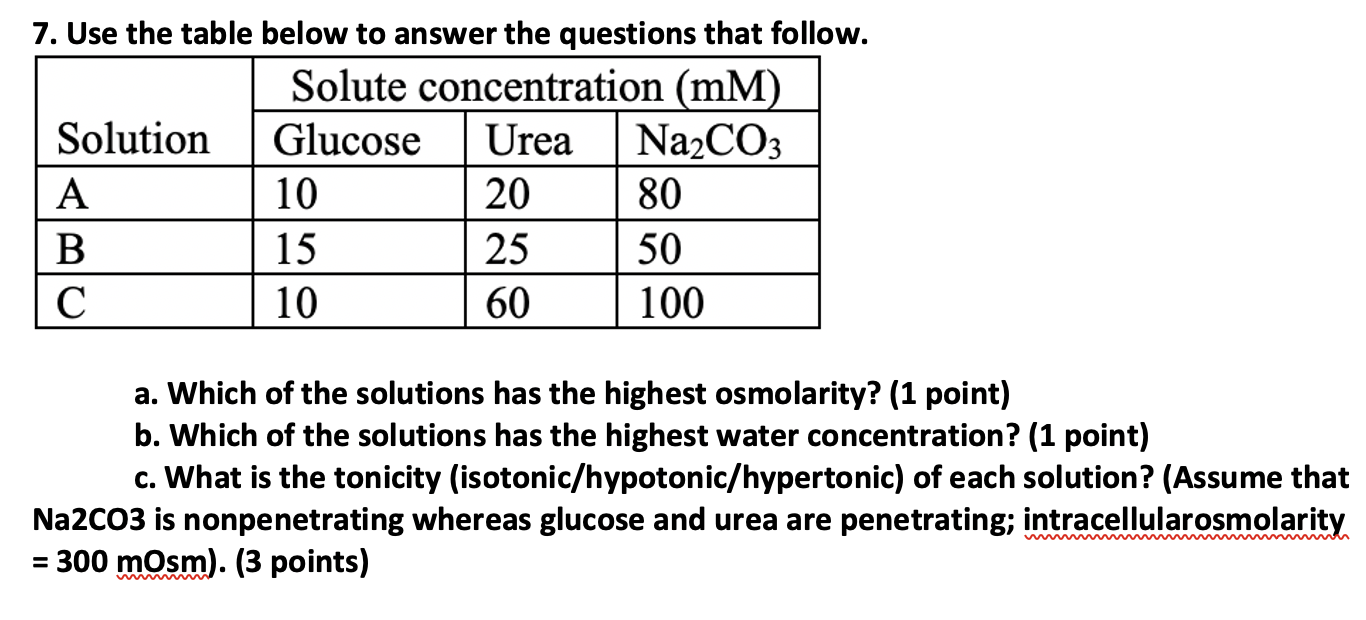
7. Use the table below to answer the questions that follow. a. Which of the solutions has the highest osmolarity? (1 point) b. Which of the solutions has the highest water concentration? (1 point) c. What is the tonicity (isotonic/hypotonic/hypertonic) of each solution? (Assume that Na2CO3 is nonpenetrating whereas glucose and urea are penetrating; intracellularosmolarity =300mOsm). (3 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


