Question: 722 Questions Assignment Score Resources Check Answer Question Question of A hydrogen om absorbs a photo of ultraviolet light exciting an electron from energy level
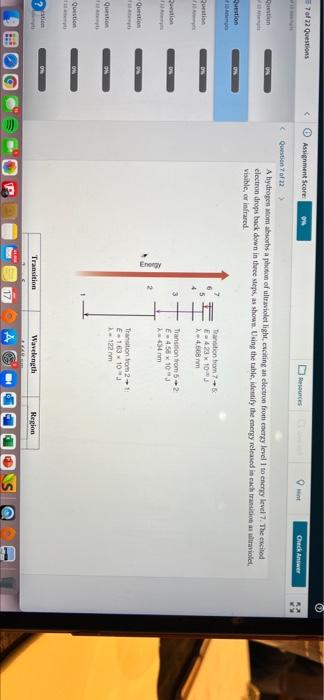
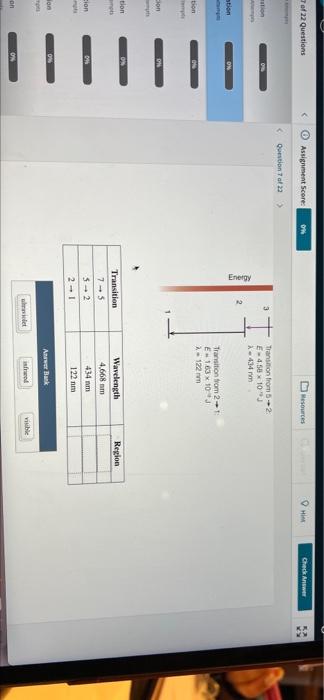
722 Questions Assignment Score Resources Check Answer Question Question of A hydrogen om absorbs a photo of ultraviolet light exciting an electron from energy level 1 to energy level 7. The excited electron drops back down in three steps, as shown. Using the table, identify the energy released in each transition as ultraviolet visible, or infrared Question Quest Thanation to F62310 X 4,60 m 4 Question Transion from E-458 103 434 nm Energy 9 Transition from 2-1 E163103 122 mm Quition ti Transition Wavelength Region of 22 Questions Assignment Score Mesources HI Check Answer Question of ution 0 3 Transition from 2 E4,58 x 10 434 m Energy stion Transition from 2 E163 x 10 2-122 mm tion son DW tion Region Transition 7-5 S-2 Wavelength 4,668 mm 414 nm 122 mm cion 04 om Anwer Bank het ifred viche ON
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


