Question: 8. Ascorbic acid, or vitamin C, is a common cold remedy and a cure for scurvy. The plot below shows the titration of a
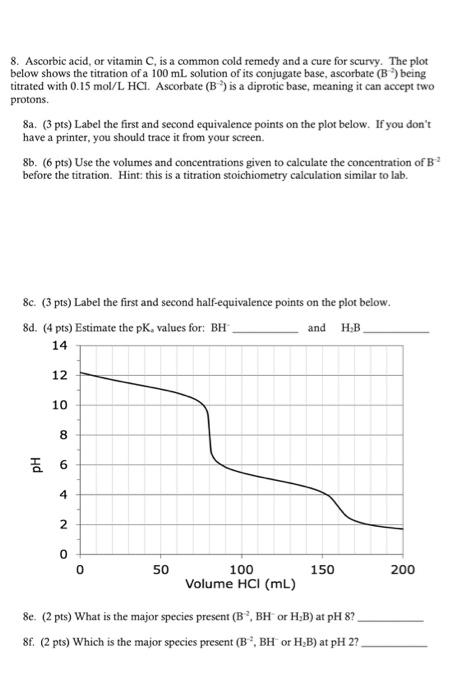
8. Ascorbic acid, or vitamin C, is a common cold remedy and a cure for scurvy. The plot below shows the titration of a 100 mL solution of its conjugate base, ascorbate (B) being titrated with 0.15 mol/L HCI. Ascorbate (B) is a diprotic base, meaning it can accept two protons. 8a. (3 pts) Label the first and second equivalence points on the plot below. If you don't have a printer, you should trace it from your screen. 8b. (6 pts) Use the volumes and concentrations given to calculate the concentration of B before the titration. Hint: this is a titration stoichiometry calculation similar to lab. 8c. (3 pts) Label the first and second half-equivalence points on the plot below. 8d. (4 pts) Estimate the pK, values for: BH and H-B 14 112 12 10 10 00 8 6 4 2 50 100 150 200 Volume HCI (mL) 8e. (2 pts) What is the major species present (B, BH or H,B) at pH 8? 8f. (2 pts) Which is the major species present (B, BH or H,B) at pH 27.
Step by Step Solution
There are 3 Steps involved in it
SOLUTION 8a Equivalence point is that point at which reaction is completed here in this case reaction is acid base reaction of conjugate base of ascor... View full answer

Get step-by-step solutions from verified subject matter experts


