Question: 8- a) b) C) d) When 1.57 mol O2 reacts with H2 to form H2O, how many moles of H2 are consumed in the
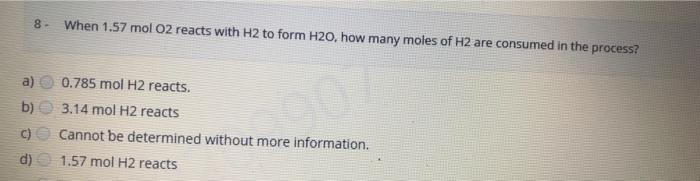
8- a) b) C) d) When 1.57 mol O2 reacts with H2 to form H2O, how many moles of H2 are consumed in the process? 0.785 mol H2 reacts. 3.14 mol H2 reacts Cannot be determined without more information. 1.57 mol H2 reacts
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Given 157 moles 02 reacts with H2 to form H20 So The chemical reaction is H2 02 H2... View full answer

Get step-by-step solutions from verified subject matter experts


