Question: 8. Carbon has two solid phases (graphite, diamond), one liquid phase, and one gas phase (5 pts). 1) Identify each of the phases of carbon
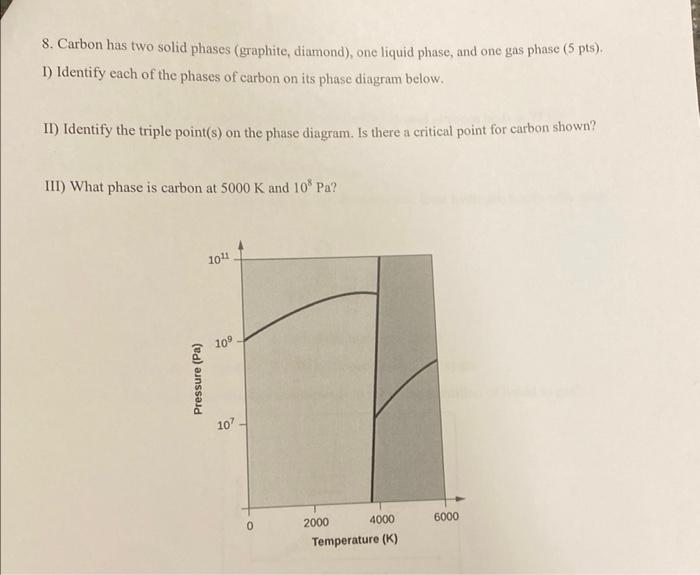
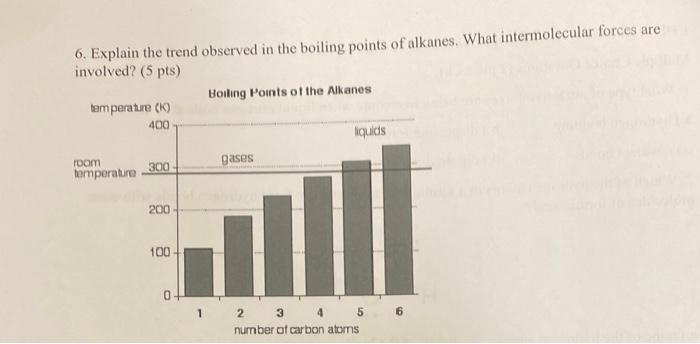
8. Carbon has two solid phases (graphite, diamond), one liquid phase, and one gas phase (5 pts). 1) Identify each of the phases of carbon on its phase diagram below. II) Identify the triple point(s) on the phase diagram. Is there a critical point for carbon shown? III) What phase is carbon at 5000K and 108Pa ? 6. Explain the trend observed in the boiling points of alkanes. What intermolecular forces are involved? (5 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


