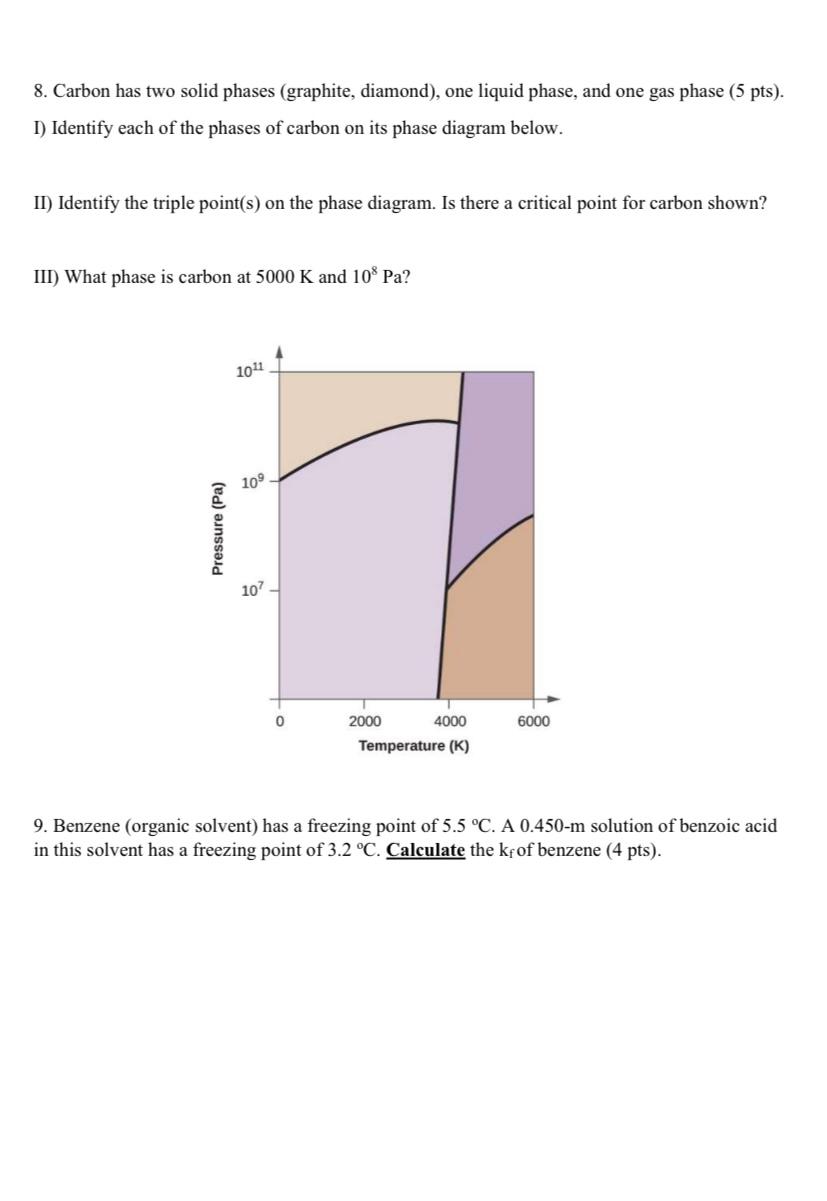
Question: help 8. Carbon has two solid phases (graphite, diamond), one liquid phase, and one gas phase (5 pts). I) Identify each of the phases of
 help
help
8. Carbon has two solid phases (graphite, diamond), one liquid phase, and one gas phase (5 pts). I) Identify each of the phases of carbon on its phase diagram below. II) Identify the triple point(s) on the phase diagram. Is there a critical point for carbon shown? III) What phase is carbon at 5000K and 108Pa ? 9. Benzene (organic solvent) has a freezing point of 5.5C. A 0.450m solution of benzoic acid in this solvent has a freezing point of 3.2C. Calculate the kf of benzene (4 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


