Question: 8) Fill in the ? with the missing product or reagents. H2SO4HNO3 ? FeCl3Cl2 ? 2) 6) Give the major product(s) and mechanism for the
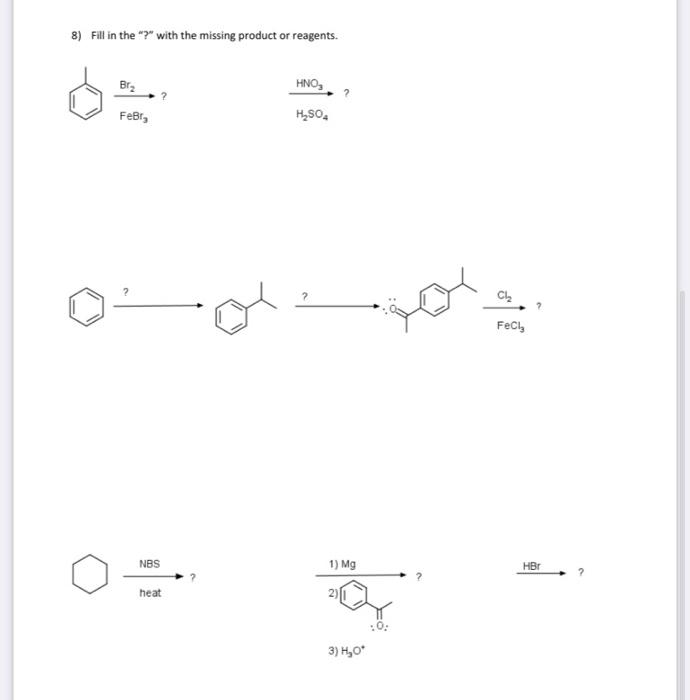

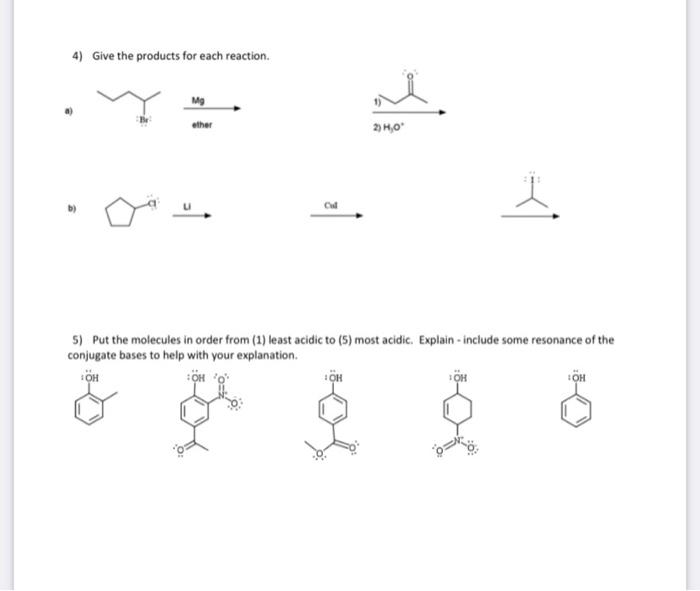
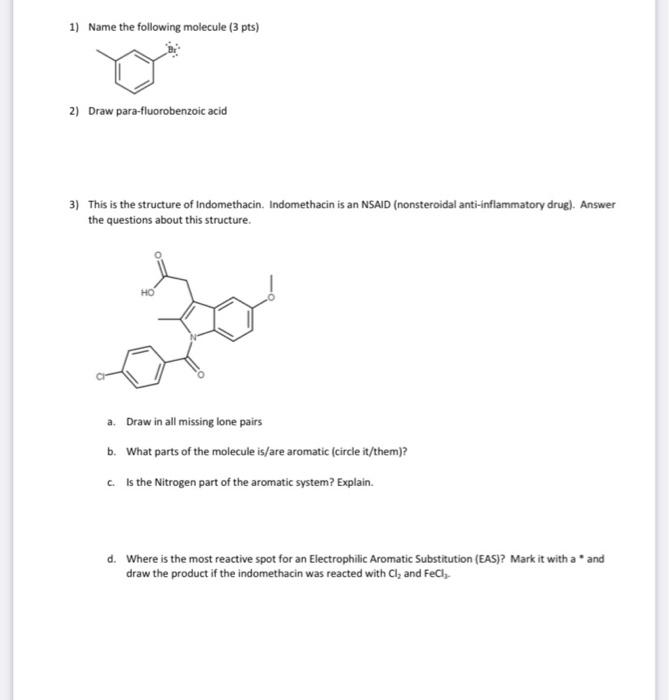
8) Fill in the "?" with the missing product or reagents. H2SO4HNO3 ? FeCl3Cl2 ? 2) 6) Give the major product(s) and mechanism for the following reaction (if more than one major product, show the mechanism to just one). Include the mechanism to make the electrophile. Be sure to include all important resonance structures. 7) Synthesis! Give a viable synthesis for the following target molecule from the starting molecule. Give reactants and products for every step. No mechanisms are needed. Double check your work for any easier route! 4) Give the products for each reaction. a) b) 5) Put the molecules in order from (1) least acidic to (5) most acidic. Explain - include some resonance of the conjugate bases to help with your explanation. 1) Name the following molecule ( 3 pts) 2) Draw para-fluorobenzoic acid 3) This is the structure of Indomethacin. Indomethacin is an NSAID (nonsteroidal anti-inflammatory drug). Answer the questions about this structure. a. Draw in all missing lone pairs b. What parts of the molecule is/are aromatic (circle it/them)? c. Is the Nitrogen part of the aromatic system? Explain. d. Where is the most reactive spot for an Electrophilic Aromatic Substitution (EAS)? Mark it with a and draw the product if the indomethacin was reacted with Cl2 and FeCl3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


