Question: 8 points total a. Draw the following structure and fill in any missing formal charges. (2 points) b. Then, draw the most stable resonance structure
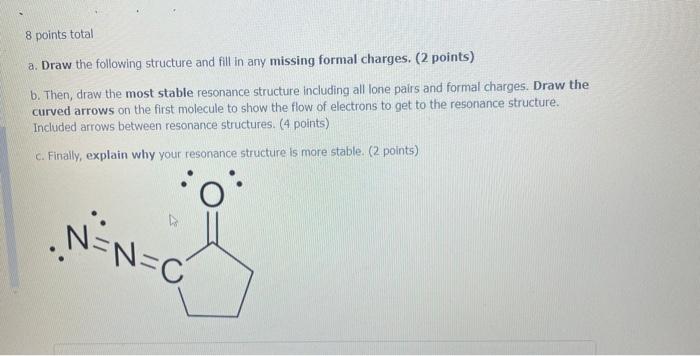
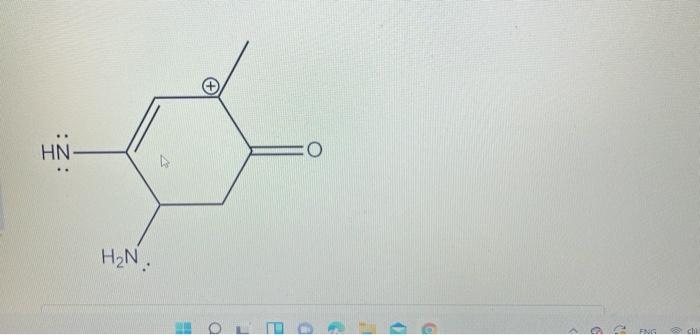
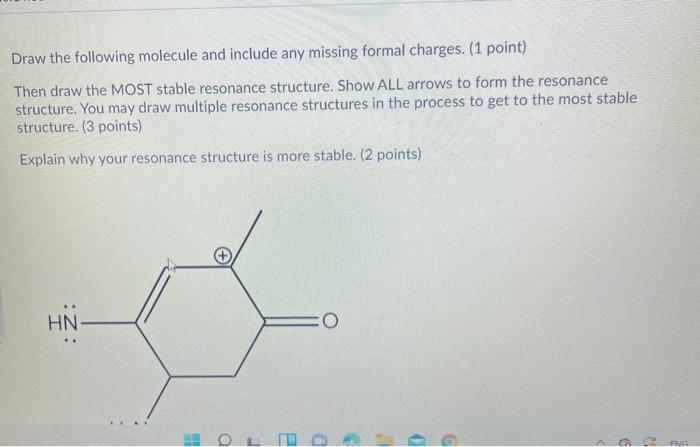
8 points total a. Draw the following structure and fill in any missing formal charges. (2 points) b. Then, draw the most stable resonance structure including all lone pairs and formal charges. Draw the curved arrows on the first molecule to show the flow of electrons to get to the resonance structure. Included arrows between resonance structures. (4 points) c. Finally, explain why your resonance structure is more stable. (2 points) N=N=C HN H2N. O a 9 FR Draw the following molecule and include any missing formal charges. (1 point) Then draw the MOST stable resonance structure. Show ALL arrows to form the resonance structure. You may draw multiple resonance structures in the process to get to the most stable structure. (3 points) Explain why your resonance structure is more stable. (2 points) HN O : HOL D - 9 c
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


