Question: 8. Propose a structure to go along with the spectrum below assuming the unknown has molecular formula C.HgO, and the integrations are as shown. (Hint:
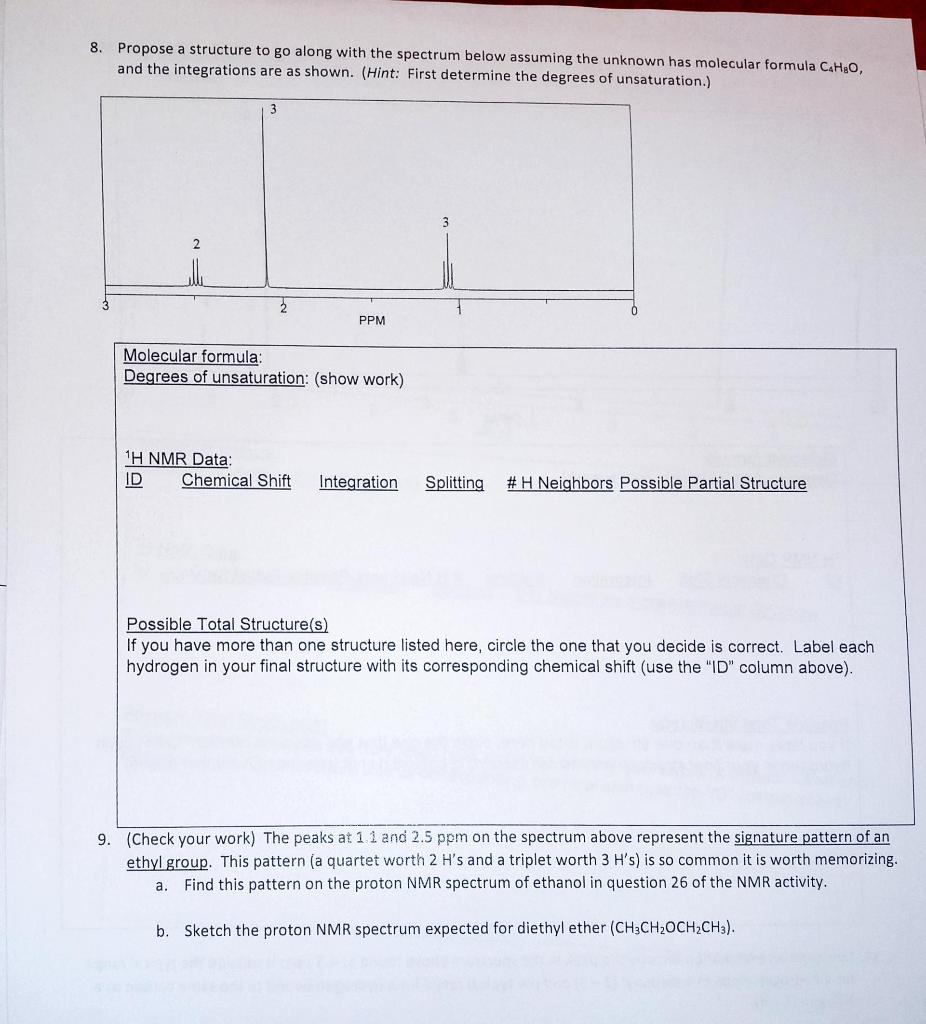
8. Propose a structure to go along with the spectrum below assuming the unknown has molecular formula C.HgO, and the integrations are as shown. (Hint: First determine the degrees of unsaturation.) 2 2 6 PPM Molecular formula: Degrees of unsaturation: (show work) 1H NMR Data: ID Chemical Shift Integration Splitting # H Neighbors Possible Partial Structure Possible Total Structure(s) If you have more than one structure listed here, circle the one that you decide is correct. Label each hydrogen in your final structure with its corresponding chemical shift (use the "ID" column above). 9. (Check your work) The peaks at 11 and 2.5 ppm on the spectrum above represent the signature pattern of an ethyl group. This pattern (a quartet worth 2 H's and a triplet worth 3 H's) is so common it is worth memorizing. a. Find this pattern on the proton NMR spectrum of ethanol in question 26 of the NMR activity. b. Sketch the proton NMR spectrum expected for diethyl ether (CH3CH2OCH2CH3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


