Question: 8. Using the in problem 7, if 2.50g K_{f}; CuSO 4 are dissolved in 900. mL of 0.30M N*H_{3} what are the concentrations of
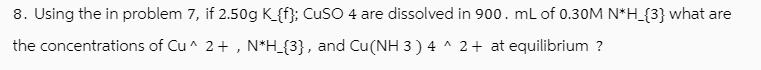
8. Using the in problem 7, if 2.50g K_{f}; CuSO 4 are dissolved in 900. mL of 0.30M N*H_{3} what are the concentrations of Cu 2+, N*H_{3}, and Cu(NH 3 ) 4^2+ at equilibrium ?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


