Question: 9 . 2 1 . If 4 4 . 9 7 m l of a soln. of H C l are equivalent to 4 3
If of a soln. of are equivalent to of a soln. of NaOH, and if of the latter will neutralize of what vol. of water should be added to a liter of the in order to make it The answer should be mL
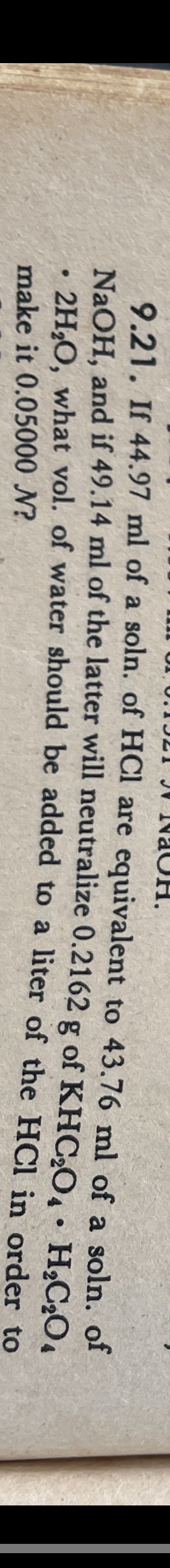
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


