Question: 9. (9 pts) We're back to interconverting normal pentane or n-pentane, isopentane, and nennentane. This time, however, our reaction conditions are insufficient to convert n-pentane
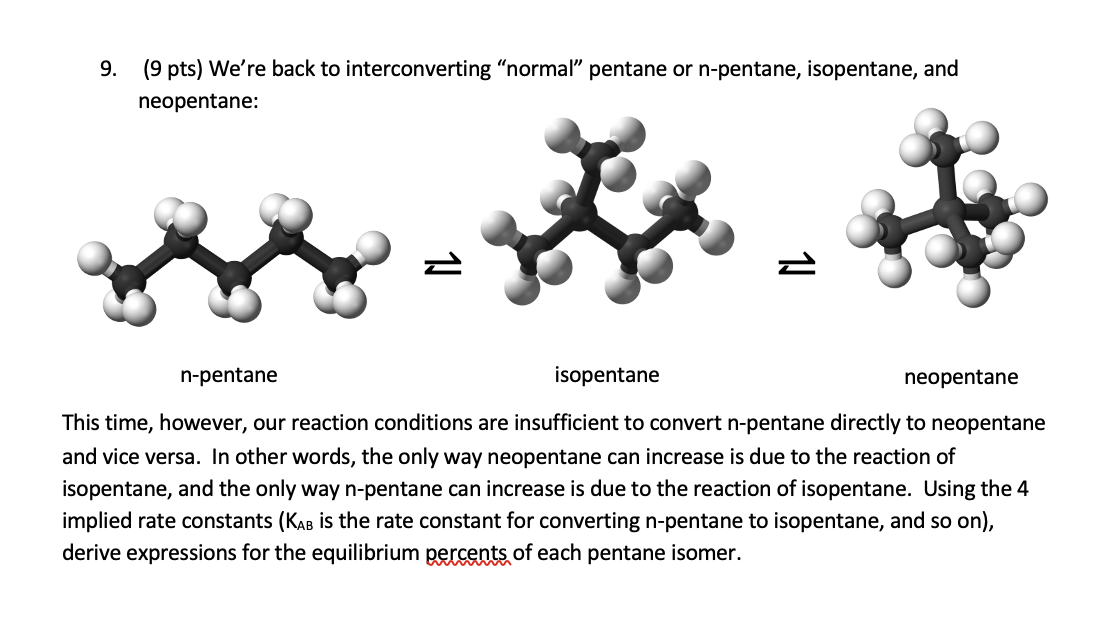
9. (9 pts) We're back to interconverting "normal" pentane or n-pentane, isopentane, and nennentane. This time, however, our reaction conditions are insufficient to convert n-pentane directly to neopentane and vice versa. In other words, the only way neopentane can increase is due to the reaction of isopentane, and the only way n-pentane can increase is due to the reaction of isopentane. Using the 4 implied rate constants ( KAB is the rate constant for converting n-pentane to isopentane, and so on), derive expressions for the equilibrium percents of each pentane isomer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


