Question: 9. Consider the Lewis structure shown below. a. What is the hybridization of the circled carbon atom? b. What is the hybridization of the circled
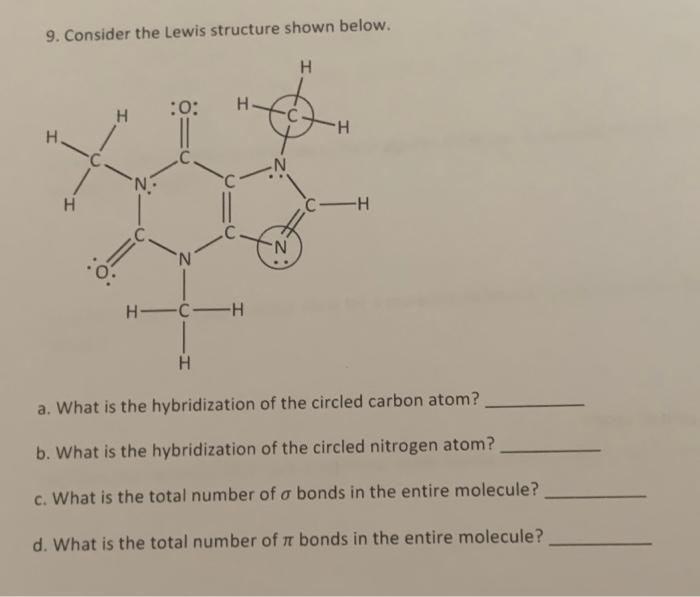
9. Consider the Lewis structure shown below. a. What is the hybridization of the circled carbon atom? b. What is the hybridization of the circled nitrogen atom? c. What is the total number of bonds in the entire molecule? d. What is the total number of bonds in the entire molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


