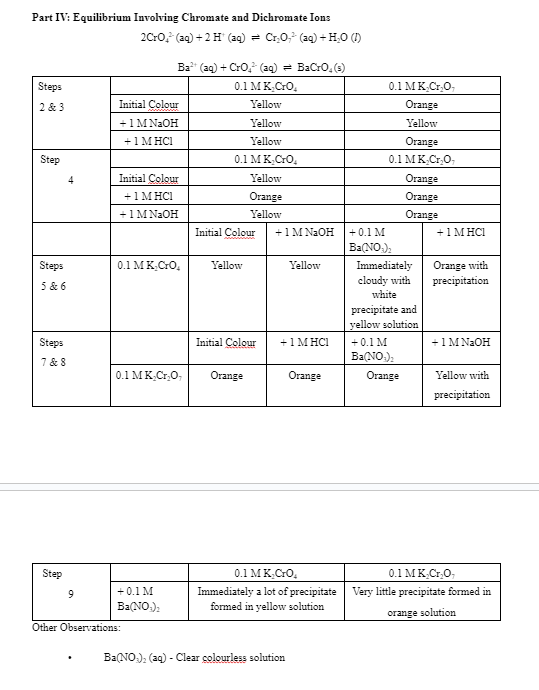
Question: 9 . Explain why no precipitate formed in K 2 Cr 2 O 7 in Step 7 of Part IV , while some did form
Explain why no precipitate formed in KCrO in Step of Part IV while some did form in Step
Part IV: Equilibrium Involving Chromate and Dichromate Ions
Other Observations:
Clear golourless solution
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


