Question: a 1. The two liquid phase reactions are taking place in a semi batch reactor. Pure feed of A with mol concentration 0.5 is fed
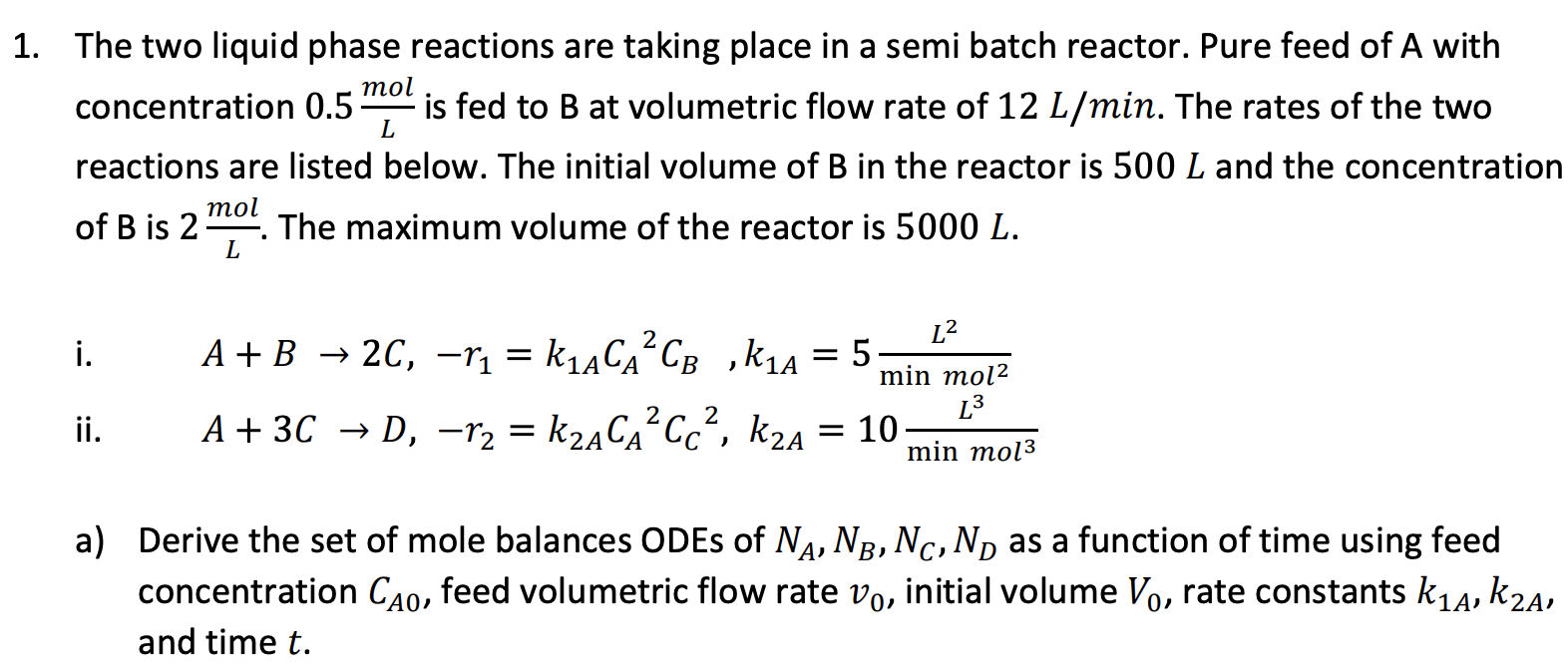
a 1. The two liquid phase reactions are taking place in a semi batch reactor. Pure feed of A with mol concentration 0.5 is fed to B at volumetric flow rate of 12 L/min. The rates of the two reactions are listed below. The initial volume of B in the reactor is 500 L and the concentration of B is 2 The maximum volume of the reactor is 5000 L. L mol L 2 i. = A + B + 2C, -r1 = k1ACACp , k1A A+ 3C D, r2 = kzACCc?, kua = 10 D L2 5 min mol2 L min mol3 2 ii. = A) a) Derive the set of mole balances ODEs of N, N3, Nc, Nas a function of time using feed concentration Cao, feed volumetric flow rate vo, initial volume Vo, rate constants kia, k2A, and time t. a 1. The two liquid phase reactions are taking place in a semi batch reactor. Pure feed of A with mol concentration 0.5 is fed to B at volumetric flow rate of 12 L/min. The rates of the two reactions are listed below. The initial volume of B in the reactor is 500 L and the concentration of B is 2 The maximum volume of the reactor is 5000 L. L mol L 2 i. = A + B + 2C, -r1 = k1ACACp , k1A A+ 3C D, r2 = kzACCc?, kua = 10 D L2 5 min mol2 L min mol3 2 ii. = A) a) Derive the set of mole balances ODEs of N, N3, Nc, Nas a function of time using feed concentration Cao, feed volumetric flow rate vo, initial volume Vo, rate constants kia, k2A, and time t
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


