Question: A 1.00 liter solution contains 0.26M potassium nitrite and 0.34M nitrous acid. If 0.085 moles of calcium hydroxide are added to this system, indicate whether
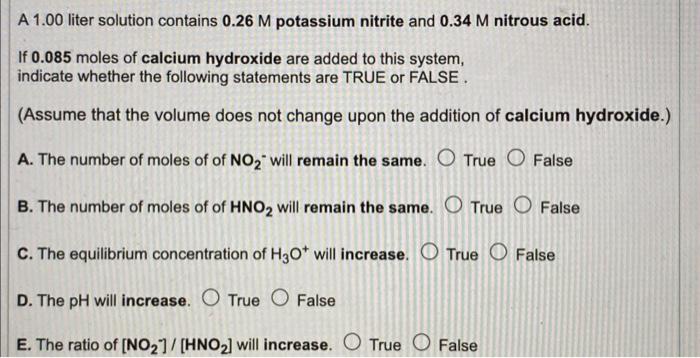
A 1.00 liter solution contains 0.26M potassium nitrite and 0.34M nitrous acid. If 0.085 moles of calcium hydroxide are added to this system, indicate whether the following statements are TRUE or FALSE. (Assume that the volume does not change upon the addition of calcium hydroxide.) A. The number of moles of of NO2 - will remain the same. True False B. The number of moles of of HNO2 will remain the same. True False C. The equilibrium concentration of H3O+will increase. True False D. The pH will increase. True False E. The ratio of [NO2]/[HNO2] will increase. True False
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


