Question: A 100mL polymerization reaction mixture was prepared from 10g methyl methacrylate and 0,1g benzoyl peroxide dissolved in benzene at 60C. Calculate the time needed to
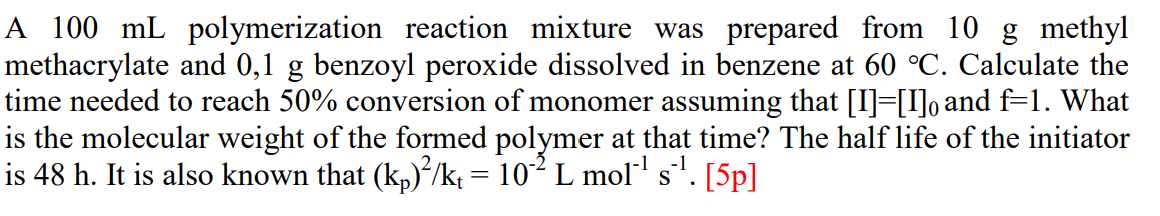
A 100mL polymerization reaction mixture was prepared from 10g methyl methacrylate and 0,1g benzoyl peroxide dissolved in benzene at 60C. Calculate the time needed to reach 50% conversion of monomer assuming that [I]=[I]0 and f=1. What is the molecular weight of the formed polymer at that time? The half life of the initiator is 48h. It is also known that (kp)2/kt=102Lmol1s1. [5p]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


