Question: a. Derive an expression for b. The initial production rate of R is measured for various concentrations of CA and Cg. In the first
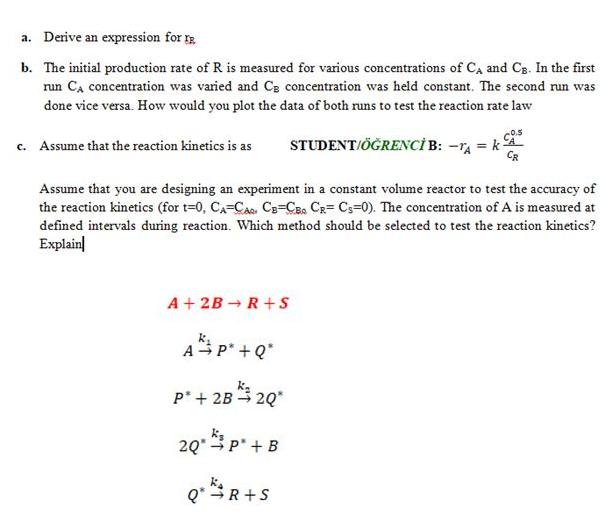
a. Derive an expression for b. The initial production rate of R is measured for various concentrations of CA and Cg. In the first run Ca concentration was varied and CB concentration was held constant. The second run was done vice versa. How would you plot the data of both runs to test the reaction rate law c. Assume that the reaction kinetics is as STUDENT/RENC B: -rA = kA Assume that you are designing an experiment in a constant volume reactor to test the accuracy of the reaction kinetics (for t=0, CA=CA. C3=CB CR= Cs=0). The concentration of A is measured at defined intervals during reaction. Which method should be selected to test the reaction kinetics? Explain A + 2B R+s A P*+Q* P* + 2B 2Q* 20.p' + B Q'R+S
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


