Question: A 403mL hydrochloric acid ( HCl) solution reacts completely with sodium carbonate (Na2CO3), forming 20.1g of carbon dioxide (CO2) according to the equation: 2HCl(aq)+Na2CO3(aq)2NaCl(aq)+H2O(l)+CO2(g) What
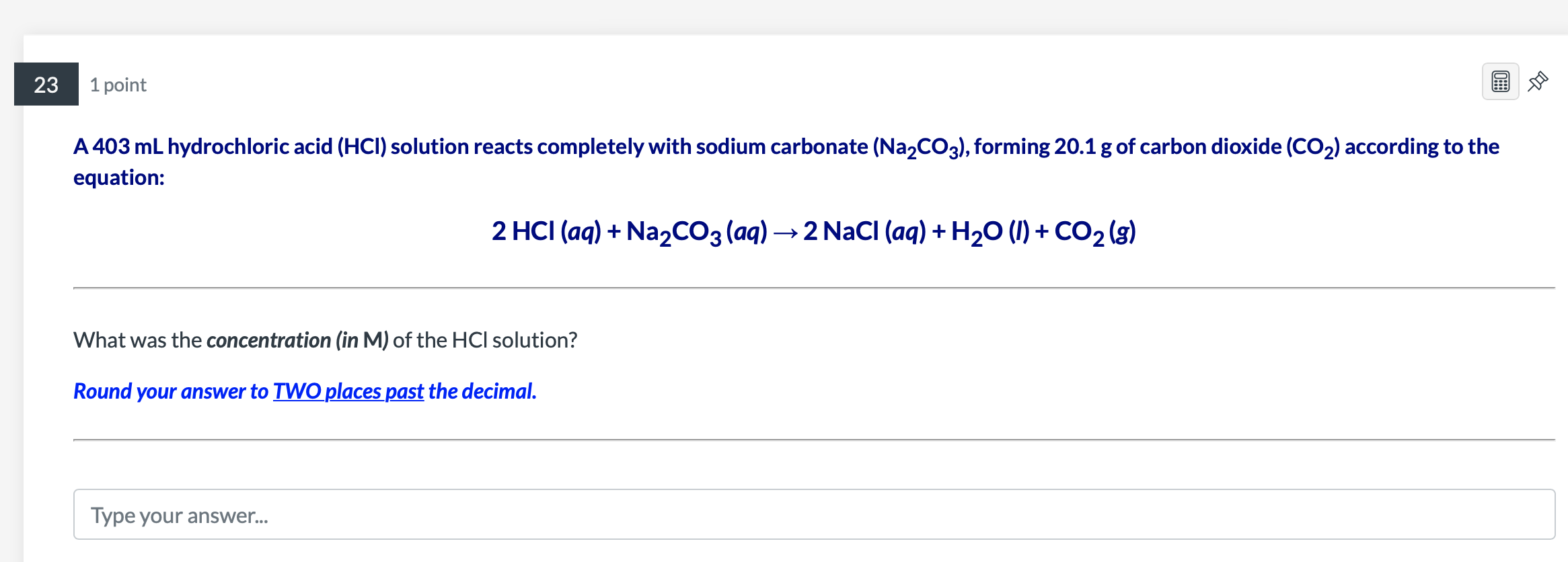
A 403mL hydrochloric acid ( HCl) solution reacts completely with sodium carbonate (Na2CO3), forming 20.1g of carbon dioxide (CO2) according to the equation: 2HCl(aq)+Na2CO3(aq)2NaCl(aq)+H2O(l)+CO2(g) What was the concentration (in M ) of the HCl solution? Round your answer to TWO places past the decimal
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


