Question: A 4.76 sample of a solid containing Ni is dissolved in 20.0 mL water.. Determine the molar concentration of Ni in the 20.0 mL solution.
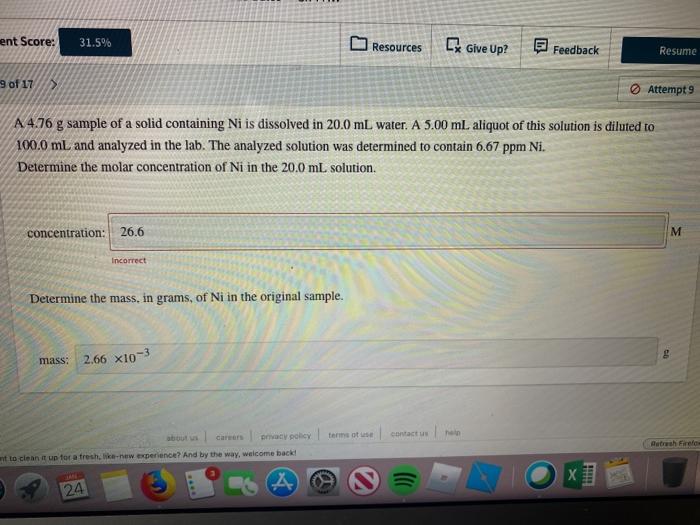
ent Score: 31.5% Resources Ex Give Up? Feedback Resume 9 of 17 > Attempt 9 A 4.76 g sample of a solid containing Ni is dissolved in 20.0 mL water. A 5.00 mL aliquot of this solution is diluted to 100.0 mL and analyzed in the lab. The analyzed solution was determined to contain 6.67 ppm Ni. Determine the molar concentration of Ni in the 20.0 mL solution. concentration: 26.6 M incorrect Determine the mass, in grams, of Ni in the original sample. mass: 2.66 X10-3 boutis laevacy policy terms of use contact us ant to clean it up for a fresh new experience? And by the way, welcome back! X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


