Question: A and B react according to: A+2Bproducts The initial amounts of A and B for several experiments are shown in the images below. A B
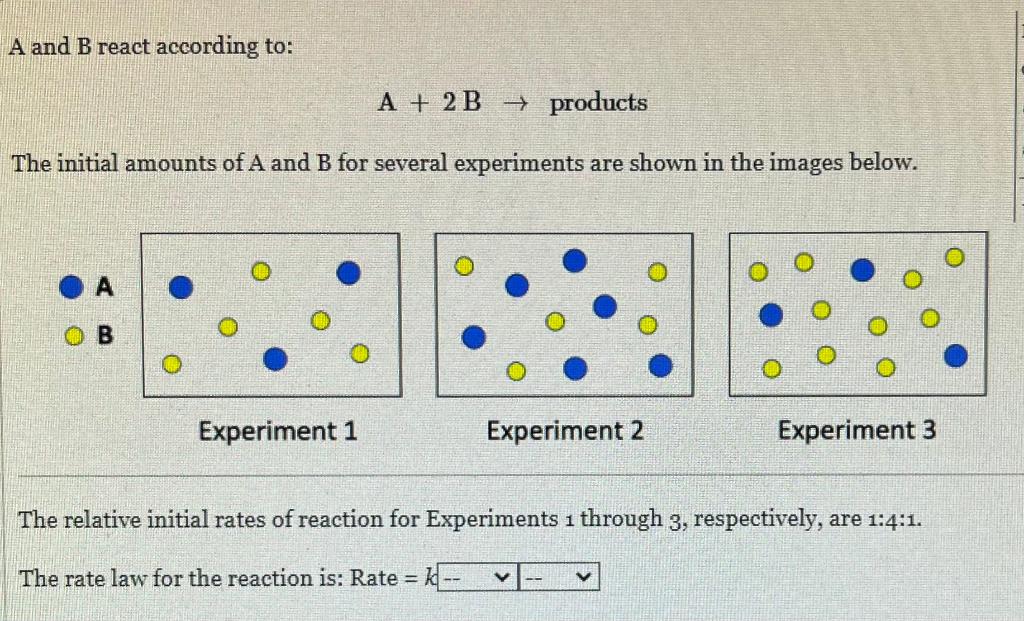

A and B react according to: A+2Bproducts The initial amounts of A and B for several experiments are shown in the images below. A B Experiment 1 Experiment 2 Experiment 3 The relative initial rates of reaction for Experiments 1 through 3 , respectively, are 1:4:1. The rate law for the reaction is: Rate =k The decomposition of HI(g) to H2(g) and I2(g) at 600K is 2nd order with a rate constant, k, of 5.82104M1min1. In an experiment the initial concentration of HI is 0.125M. What is the concentration of HI after 75.oh ? M How many hours does it take for [HI] to decrease to 0.0450M ? hr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


