Question: A and B react according to: A+2Bproducts The initial amounts of A and B for several experiments are shown in the images below. Experiment 1
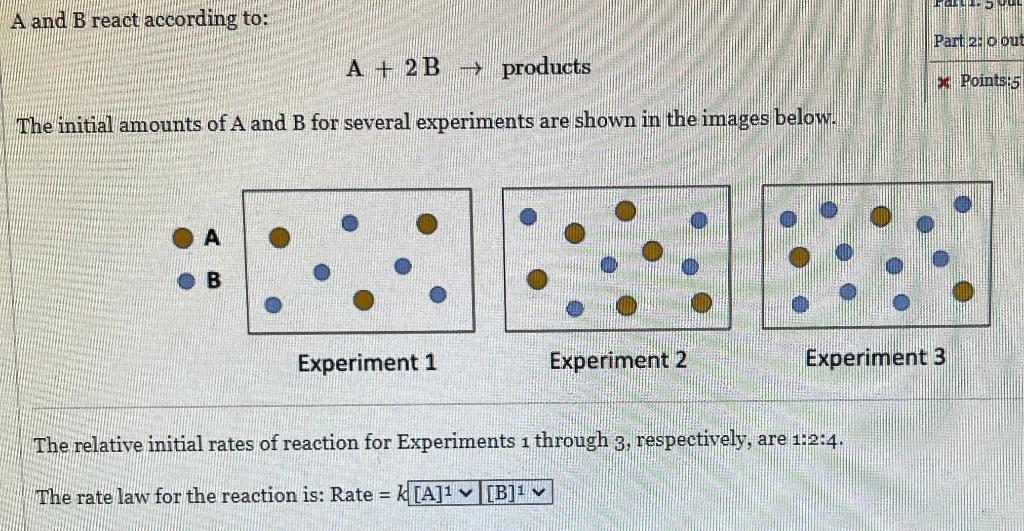
A and B react according to: A+2Bproducts The initial amounts of A and B for several experiments are shown in the images below. Experiment 1 Experiment 2 The relative initial rates of reaction for Experiments 1 through 3 , respectively, are 1:2:4. The rate law for the reaction is: Rate =k[A]1[B]1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


