Question: A basic drug with a pka value of 7.5 is dissolved in buffer of pH 5.2. If the true octanol/water partition coefficient is 135, calculate
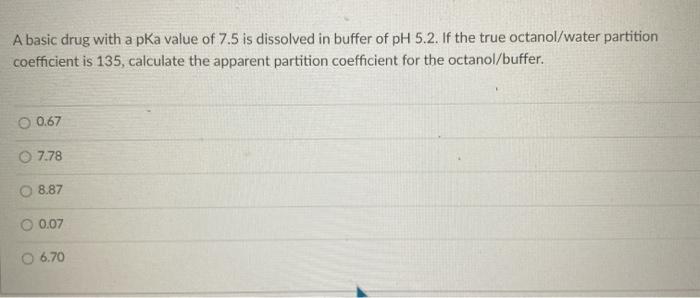
A basic drug with a pka value of 7.5 is dissolved in buffer of pH 5.2. If the true octanol/water partition coefficient is 135, calculate the apparent partition coefficient for the octanol/buffer. 0.67 7.78 O 8.87 0.07 6.70
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


