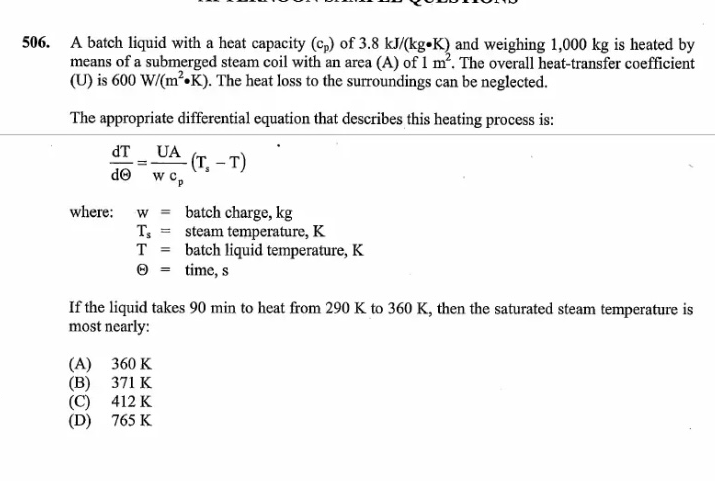
Question: A batch liquid with a heat capacity ( c p ) of 3 . 8 k J k g * K and weighing 1 ,
A batch liquid with a heat capacity of and weighing is heated by
means of a submerged steam coil with an area A of The overall heattransfer coefficient
is The heat loss to the surroundings can be neglected.
The appropriate differential equation that describes this heating process is:
where: batch charge,
steam temperature,
batch liquid temperature,
time,
If the liquid takes min to heat from to then the saturated steam temperature is
most nearly:
A
B
C
D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


