Question: A binary mixture containing component A and component B is to be separated by using a single - stage flash drum. Vapor - liquid equilibrium
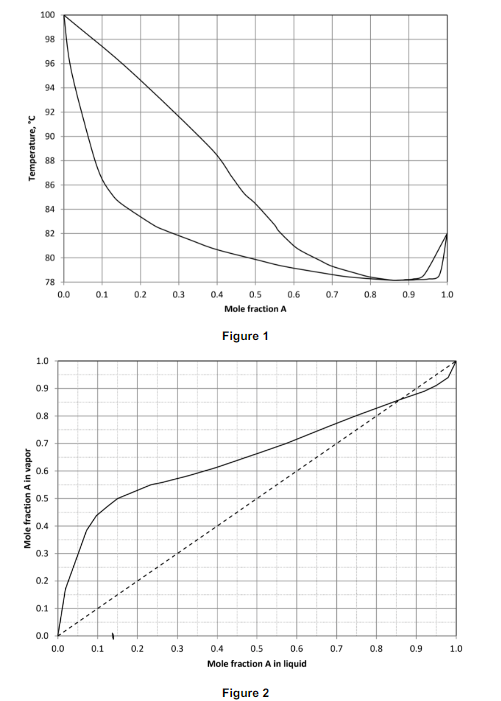
A binary mixture containing component A and component is to be separated by using a singlestage flash drum. Vaporliquid equilibrium data at atm for the AB system are provided in the
and plots in Figure and respectively. Use these data to answer the questions below.
a If the mixture contains molA and is heated to what fraction of the system remains in the liquid phase?
b A new mixture containing is heated until the system is vaporized. What are the resulting compositions of vapor and liquid?You can use either or plots to answer this question. Please specify the figure you used.
c When the mixture compositions are above mol which component is more volatile? A or B Please justify your answer
d What is the composition of azeotrope in this system? Is the azeotrope in this system minimum boiling or maximum boiling?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


