Question: A buffer of pH 5.09 is required. (a) Select from the Data Sheet the most appropriate acid/conjugate base combination. K K2 1. Acid Dissociation Constants,
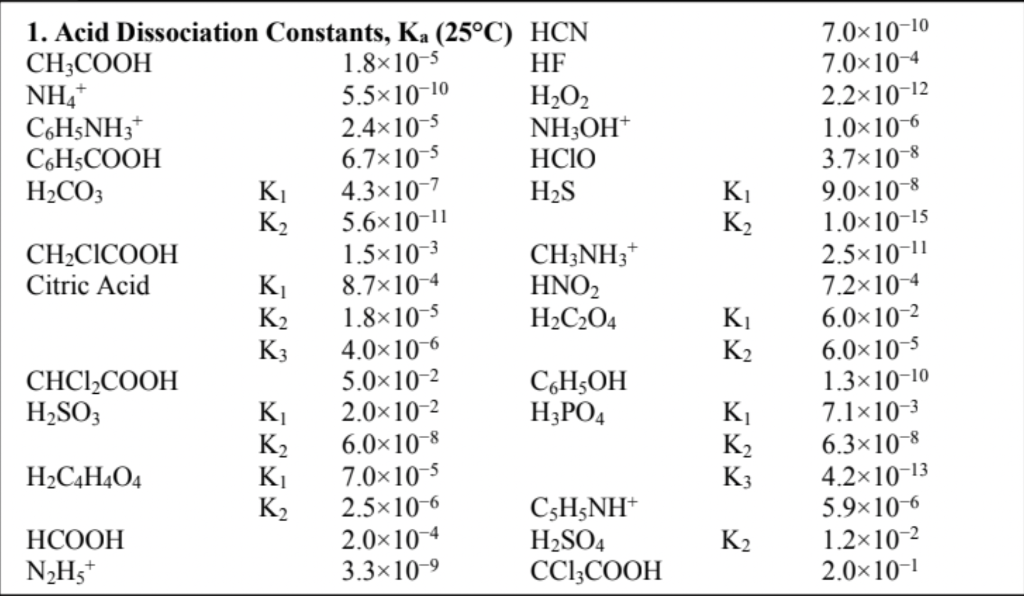
A buffer of pH 5.09 is required.
(a) Select from the Data Sheet the most appropriate acid/conjugate base combination.
K K2 1. Acid Dissociation Constants, Ka (25C) HCN CH3COOH 1.8x10-5 HF NH4 5.5x10-10 H2O2 C6H5NH3+ 2.4x10-5 NH3OH CH3COOH 6.7x10-5 HCIO H2CO3 K 4.3x10-7 H2S K2 5.6x10-11 CHCICOOH 1.5x10-3 CH3NH3 Citric Acid K 8.7x10-4 HNO2 K2 1.8x10-5 H2C204 K3 4.0x10-6 CHCI2COOH 5.0*10-2 C6H5OH H2SO3 K 2.0x10-2 H3PO4 K2 6.0x10-8 H2C4H404 K 7.0x10-5 K2 2.5x10-6 C6H5NH+ HCOOH 2.0*10-4 H2SO4 N2H5+ 3.3x10-9 CCI3COOH 7.0*10-10 7.0*10-4 2.2x10-12 1.0x106 3.7x10-8 9.0x10-8 1.0x10-15 2.5x10-11 7.2x10-4 6.0x10-2 6.0x10-5 1.3x10-10 7.1x10-3 6.3x10-8 4.2x10-13 5.9x10-6 1.2x10-2 2.0x10-1 K K2 K K2 K; K2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


