Question: A certain solid solution of A and B has a AGmix as follows: AGmix = AG mix AGmix = XBX_[a XA +a2X8]+RT[X, In XA +Xp
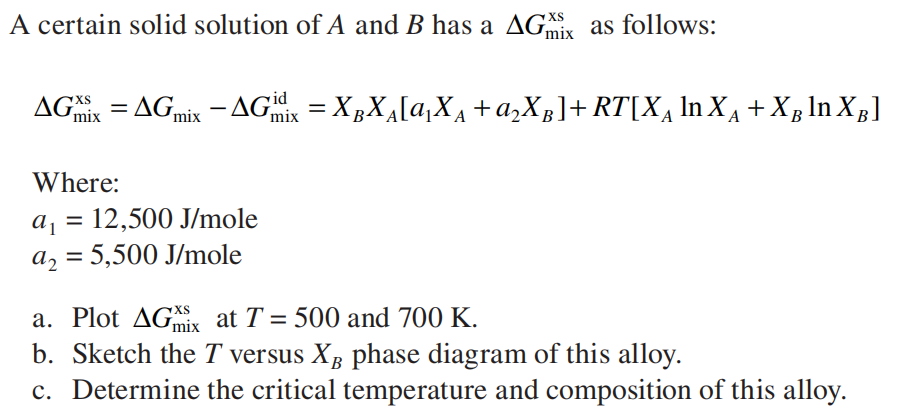
A certain solid solution of A and B has a AGmix as follows: AGmix = AG mix AGmix = XBX_[a XA +a2X8]+RT[X, In XA +Xp In Xb] Where: a = 12,500 J/mole az = 5,500 J/mole = = = a. Plot AGmix at T = 500 and 700 K. b. Sketch the T versus X8 phase diagram of this alloy. c. Determine the critical temperature and composition of this alloy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


