Question: Use data from Table 19.1, as necessary, to predict the probable products when Pt electrodes are used in the electrolysis of (a) CuCl 2 (aq);
Use data from Table 19.1, as necessary, to predict the probable products when Pt electrodes are used in the electrolysis of
(a) CuCl2(aq);
(b) Na2SO4(aq);
(c) BaCl2(l);
(d) KOH(aq).
Table 19.1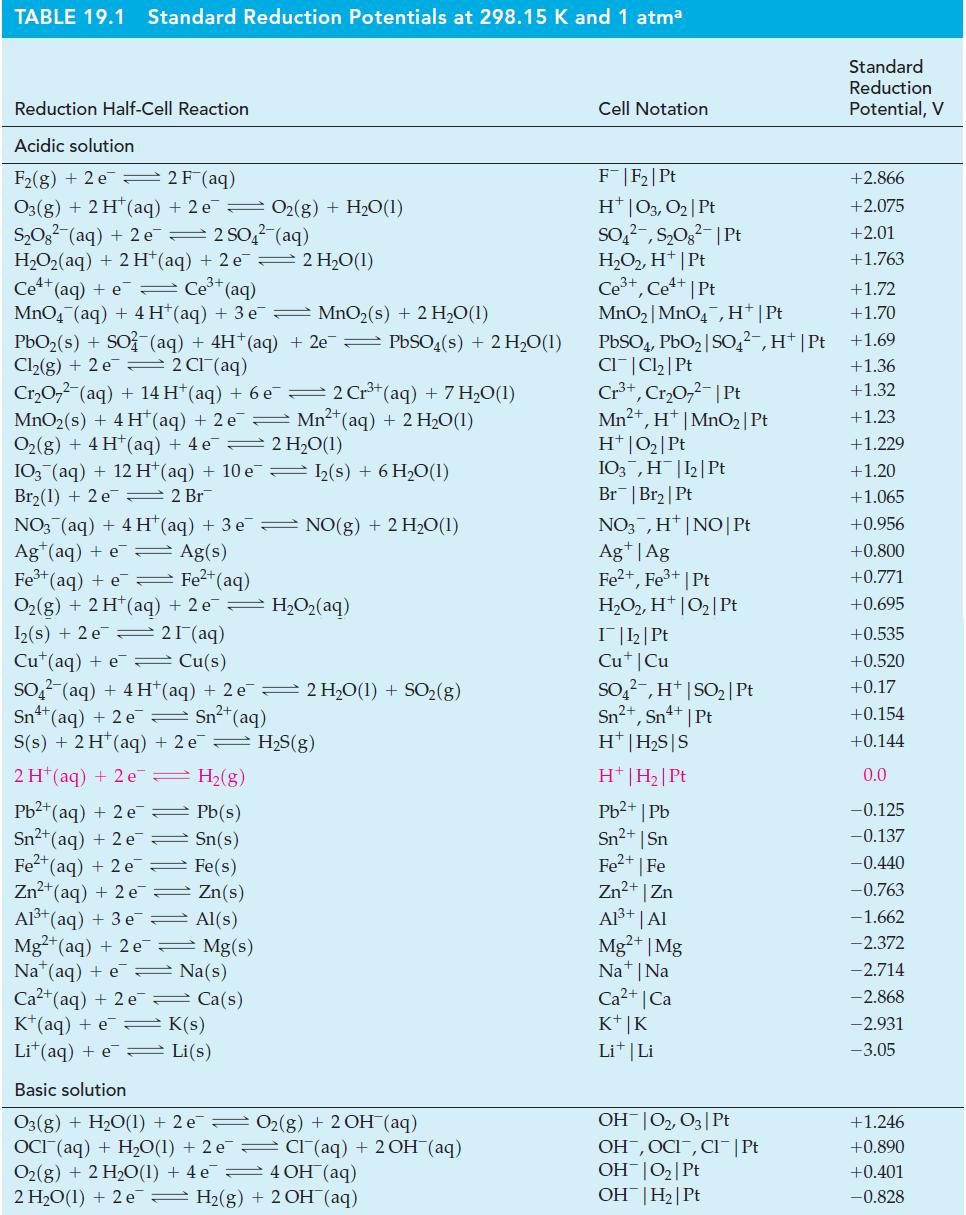
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma Reduction Half-Cell Reaction Acidic solution F(g) + 2 e 2F (aq) O3(g) + 2 H (aq) + 2 e 0(g) + HO(1) SO (aq) + 2 e 2 SO4(aq) H,Oz(aq) + 2H*(aq) +2e
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
a Electrolysis of CuCl2aq The most likely products of the electrolysis of CuCl2aq are copper metal a... View full answer

Get step-by-step solutions from verified subject matter experts


