Question: a ) Cl 2 ( aq ) + 2 Br 1 ( aq ) - > 2 Cl - ( aq ) + Br 2
a Claq Braq Claq Braq
b Claq Iaq Claq Iaq
c Braq Claq Braq Claq
d Braq Iaq Braq Iaq
e Iaq Claq Iaq Claq
f Iaq Braq Iaq Braq
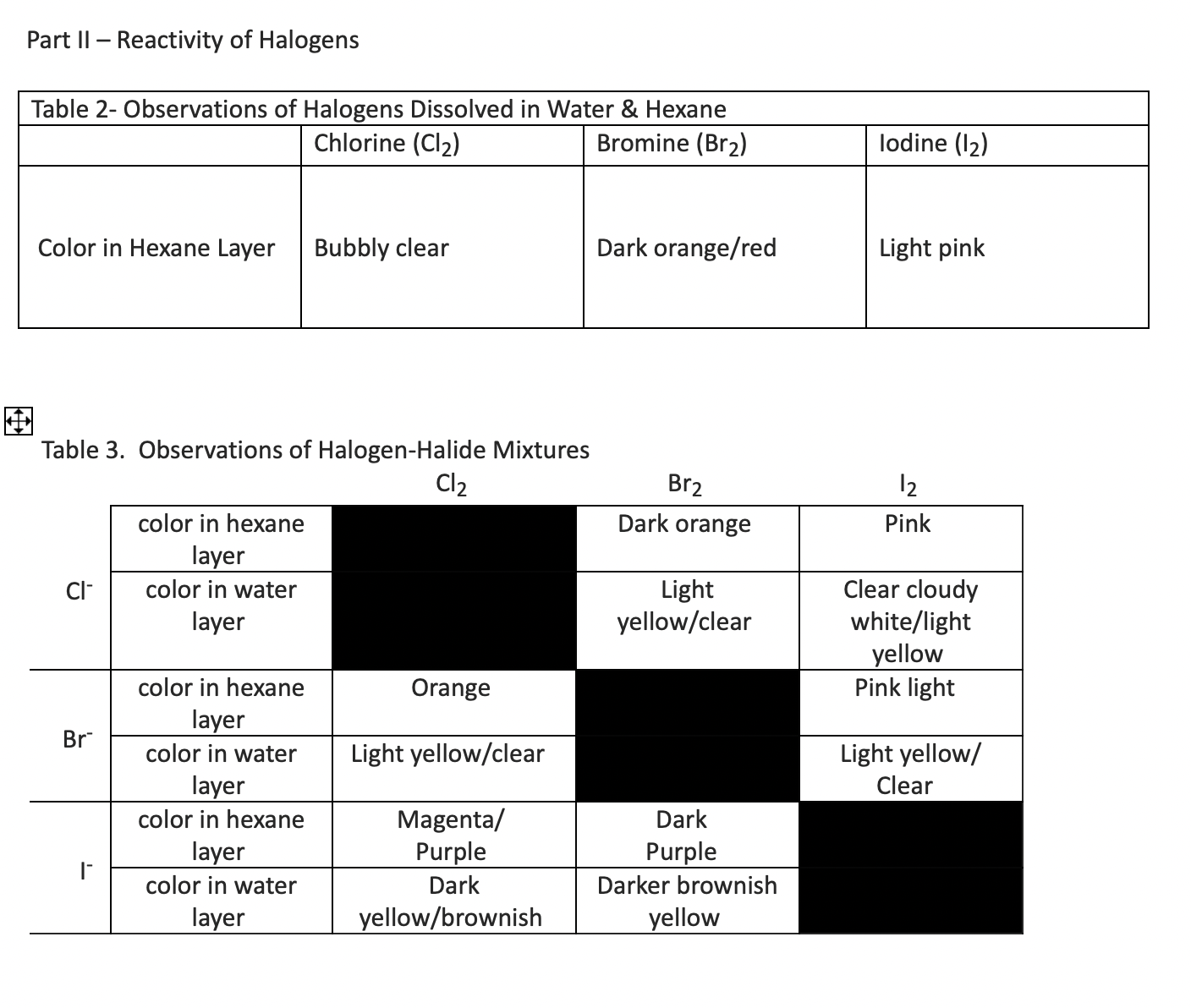
Using your observations in Tables and you will determine which of the following reaction actually did occur in the test tubes. Table gives you the color of that halogen in hexane. Looking at the results of the halogenhalide mixtures, and noting the color in the upper, hexane layer of the mixtures, underline which diatomic halogen was present in each. What do I underline based on my results for afPart II Reactivity of Halogens
Table Observations of HalogenHalide Mixtures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


