Question: A complex liquid phase reaction follows elementary rate laws and taken place in CSTR as follows; A+B+ 20 B + 2C 2D The feed stream
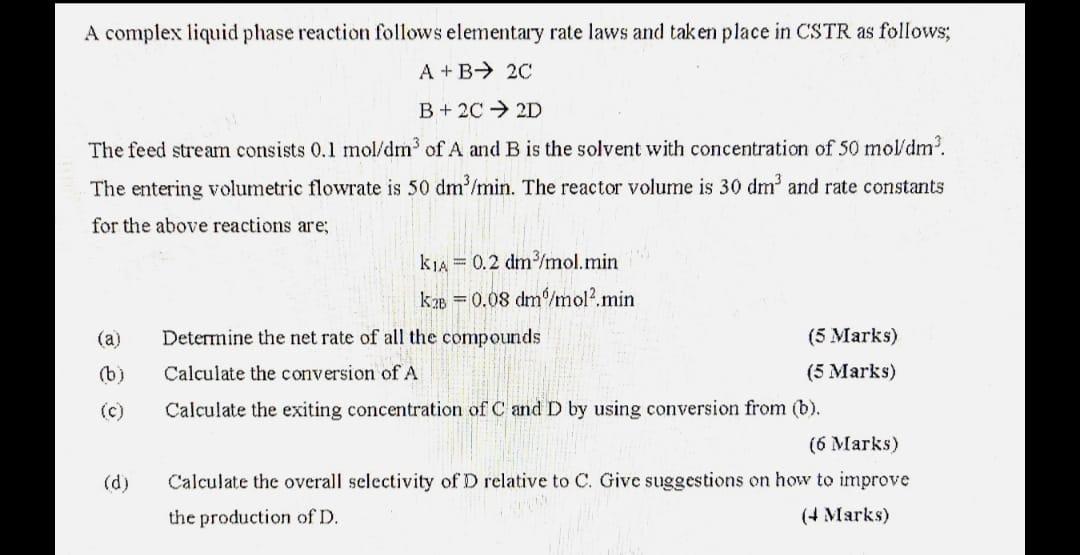
A complex liquid phase reaction follows elementary rate laws and taken place in CSTR as follows; A+B+ 20 B + 2C 2D The feed stream consists 0.1 mol/dm3 of A and B is the solvent with concentration of 50 mol/dm. The entering volumetric flowrate is 50 dm/min. The reactor volume is 30 dm' and rate constants for the above reactions are; k14 = 0.2 dm/mol.min k2 = 0.08 dm/mol2 min (a) Determine the net rate of all the compounds (5 Marks) (6) Calculate the conversion of A (5 Marks) (c) Calculate the exiting concentration of C and D by using conversion from (b). (6 Marks) (d) Calculate the overall selectivity of D relative to C. Give suggestions on how to improve the production of D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


