Question: A copper electroplating cell operates as a continuous stirred tank reactor, with copper deposition occurring at the cathode and oxygen evolution at the anode. The
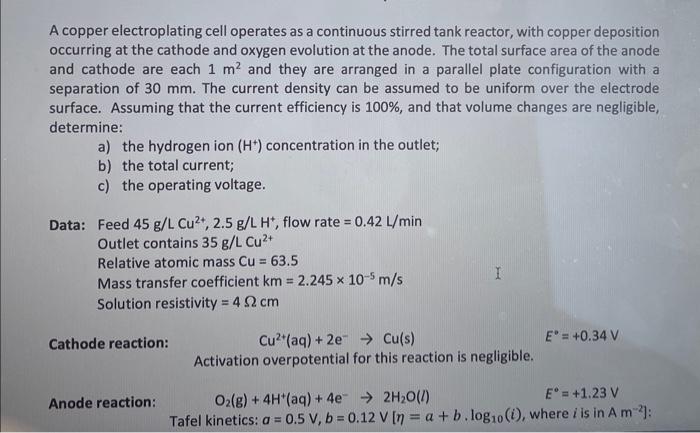
A copper electroplating cell operates as a continuous stirred tank reactor, with copper deposition occurring at the cathode and oxygen evolution at the anode. The total surface area of the anode and cathode are each 1m2 and they are arranged in a parallel plate configuration with a separation of 30mm. The current density can be assumed to be uniform over the electrode surface. Assuming that the current efficiency is 100%, and that volume changes are negligible, determine: a) the hydrogen ion (H+)concentration in the outlet; b) the total current; c) the operating voltage. Data: Feed 45g/LCu2+,2.5g/LH+, flow rate =0.42L/min Outlet contains 35g/LCu2+ Relative atomic mass Cu=63.5 Mass transfer coefficient km=2.245105m/s Solution resistivity =4cm Cathode reaction: Cu2+(aq)+2eCu(s)E=+0.34V Activation overpotential for this reaction is negligible. Anode reaction: O2(g)+4H+(aq)+4e2H2O(l)E=+1.23V Tafel kinetics: a=0.5V,b=0.12V[=a+b.log10(i), where i is in A2]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


