Question: A CSTR is fed with a solution of reactant A with the concentration of 5 kmol/m3 at a flow rate of 0.04 m3 /s. The
A CSTR is fed with a solution of reactant A with the concentration of 5 kmol/m3 at a flow rate of 0.04 m3 /s. The product mixture from the 1st CSTR is fed into a 2nd CSTR having twice the volume of the 1st CSTR. The two CSTRs in series are required to achieve an overall conversion of 60%. The reaction is first-order with a reaction constant of 0.1 s-1 (A k Product). Assuming that perfect mixing, constant liquid density, constant volumetric flow rate and operating under steady-state.
a) What is the volume of the 1st CSTR?
b) What is the inlet concentration of reactant A for the 2nd CSTR?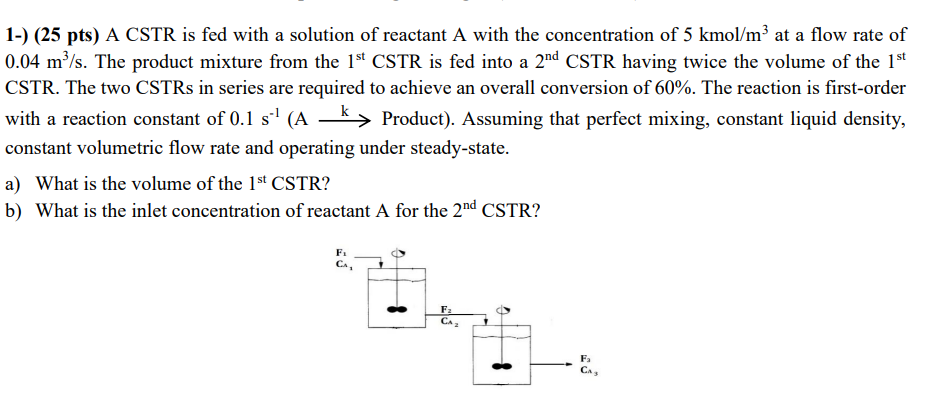
1-) (25 pts) A CSTR is fed with a solution of reactant A with the concentration of 5kmol/m3 at a flow rate of 0.04m3/s. The product mixture from the 1stCSTR is fed into a 2nd CSTR having twice the volume of the 1st CSTR. The two CSTRs in series are required to achieve an overall conversion of 60%. The reaction is first-order with a reaction constant of 0.1s1(Ak Product). Assuming that perfect mixing, constant liquid density, constant volumetric flow rate and operating under steady-state. a) What is the volume of the 1stCSTR ? b) What is the inlet concentration of reactant A for the 2nd CSTR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


