Question: Question 4 [17 Marks A CSTR is fed with a solution of reactant A of concentration 10 kmol mat a flow rate of 0.02 m's.
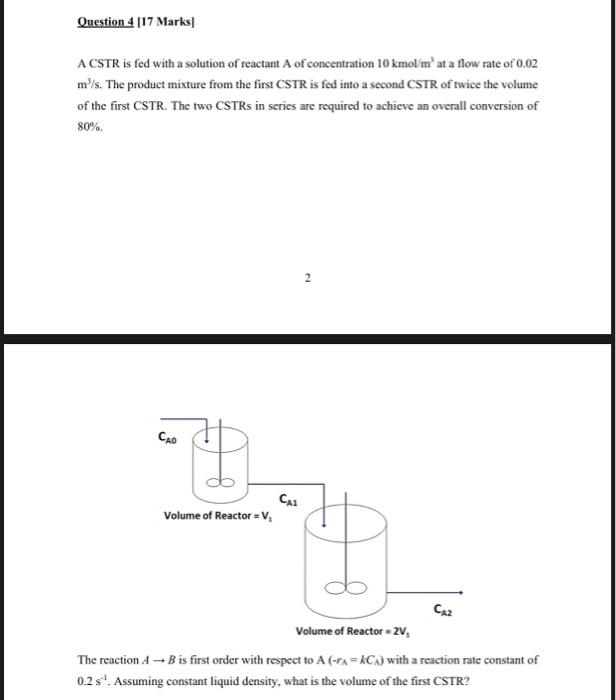
Question 4 [17 Marks A CSTR is fed with a solution of reactant A of concentration 10 kmol mat a flow rate of 0.02 m's. The product mixture from the first CSTR is fed into a second CSTR of twice the volume of the first CSTR. The two CSTRs in series are required to achieve an overall conversion of 80%. 2 2 CAO CA Volume of Reactor =V, B Caz Volume of Reactor - 2V The reaction AB is first order with respect to A (-tA=CA) with a reaction rate constant of 0.2 s'. Assuming constant liquid density, what is the volume of the first CSTR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


