Question: a. Define the following terms: i. ii. Oxidation half-reaction Reducing agent Anode iii. iv. Standard reduction potential Imk Imk 1mk 2 mks b. i.
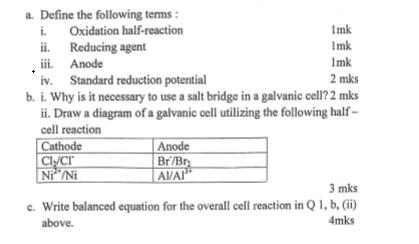
a. Define the following terms: i. ii. Oxidation half-reaction Reducing agent Anode iii. iv. Standard reduction potential Imk Imk 1mk 2 mks b. i. Why is it necessary to use a salt bridge in a galvanic cell? 2 mks ii. Draw a diagram of a galvanic cell utilizing the following half- cell reaction Cathode Cl/Cr Ni/Ni Anode Br/Br AVAI 3 mks c. Write balanced equation for the overall cell reaction in Q1, b, (ii) above. 4mks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


