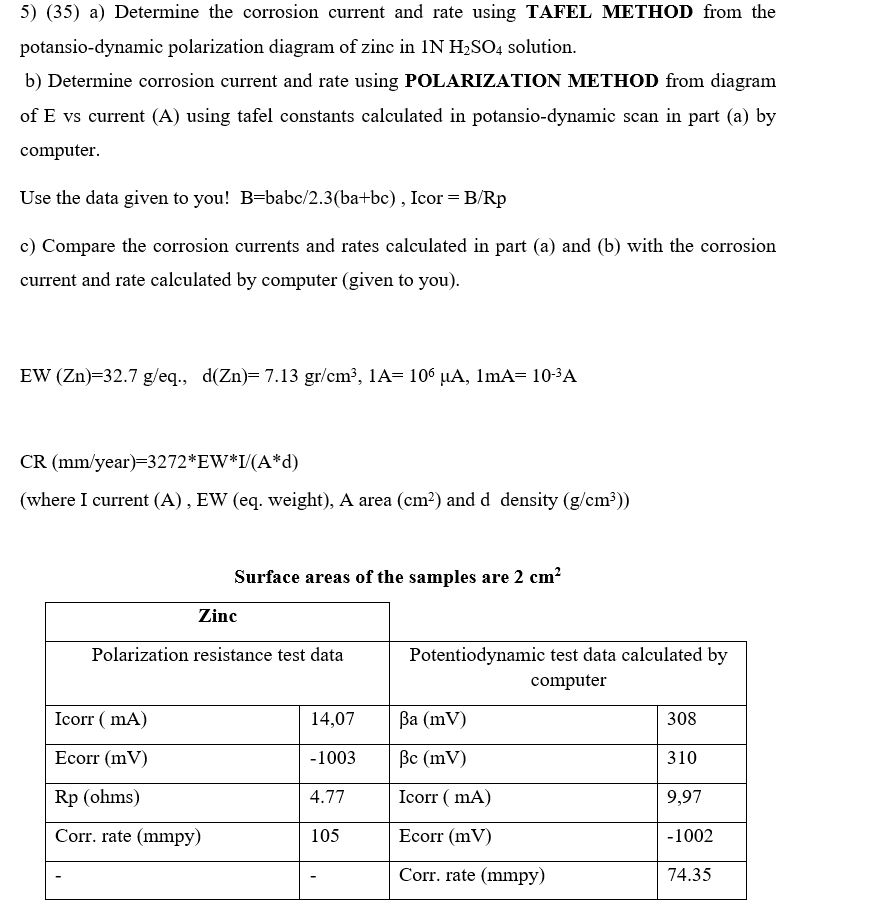
Question: a ) Determine the corrosion current and rate using TAFEL METHOD from the potansio - dynamic polarization diagram of zinc in 1 N H 2
a Determine the corrosion current and rate using TAFEL METHOD from the potansiodynamic polarization diagram of zinc in solution.
b Determine corrosion current and rate using POLARIZATION METHOD from diagram of vs current A using tafel constants calculated in potansiodynamic scan in part a by computer.
Use the data given to you! babIcor
c Compare the corrosion currents and rates calculated in part a and b with the corrosion current and rate calculated by computer given to you
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


