Question: (a) Explain by drawing resonance structures, why pyridine, pyrrole and furan are aromatic 1marks) (b) (i) What position of pyrrole more favourable to electrophillic substitution?
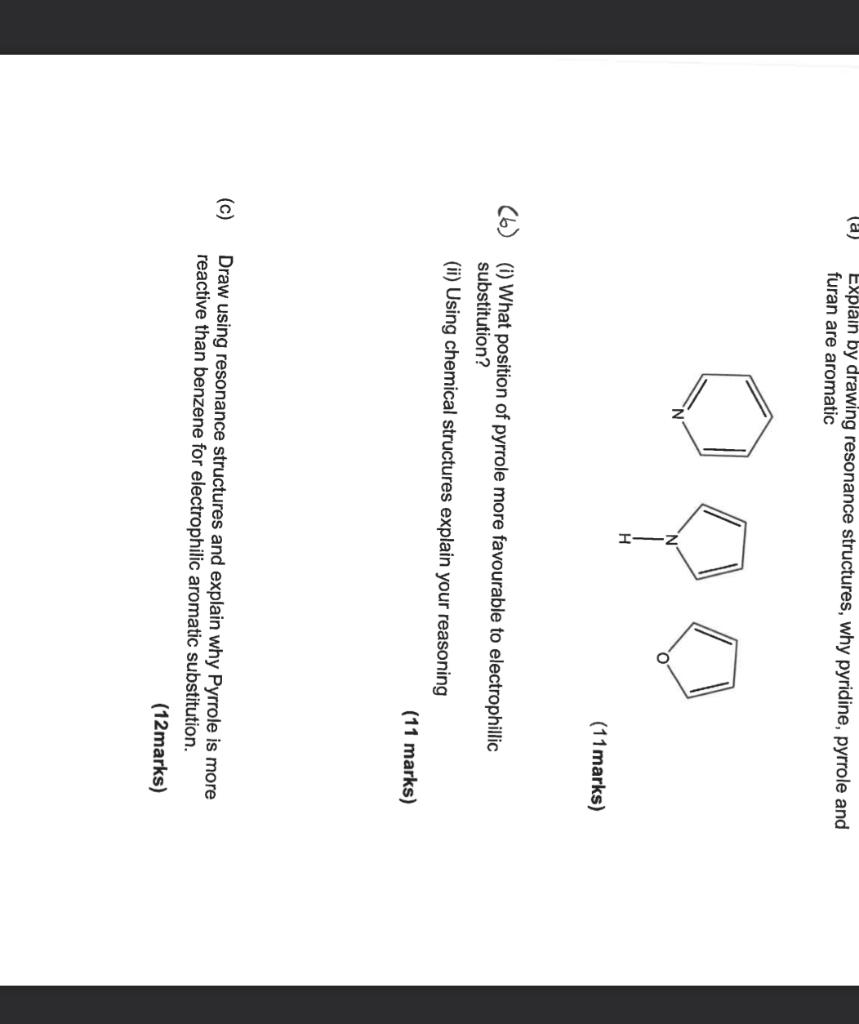
(a) Explain by drawing resonance structures, why pyridine, pyrrole and furan are aromatic 1marks) (b) (i) What position of pyrrole more favourable to electrophillic substitution? (ii) Using chemical structures explain your reasoning (11 marks) (c) Draw using resonance structures and explain why Pyrrole is more reactive than benzene for electrophilic aromatic substitution. (a) Explain by drawing resonance structures, why pyridine, pyrrole and furan are aromatic 1marks) (b) (i) What position of pyrrole more favourable to electrophillic substitution? (ii) Using chemical structures explain your reasoning (11 marks) (c) Draw using resonance structures and explain why Pyrrole is more reactive than benzene for electrophilic aromatic substitution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


