Question: (a) Find the order with respect to each reactant and find the rate constant. (6%) (b) The initial concentrations of NO and Cl2 are 0.04molL1
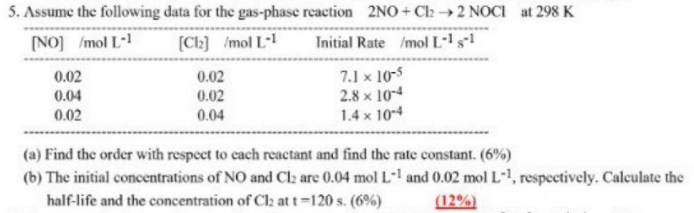
(a) Find the order with respect to each reactant and find the rate constant. (6%) (b) The initial concentrations of NO and Cl2 are 0.04molL1 and 0.02molL1, respectively. Calculate the half-life and the concentration of Cl2 at t=120s. (6\%) (12%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


