Question: A first order irreversible constant density reaction is to be carried out in the series-parallel system shown below. The reaction rate expression is rA=0.38CA,Lmingmole and
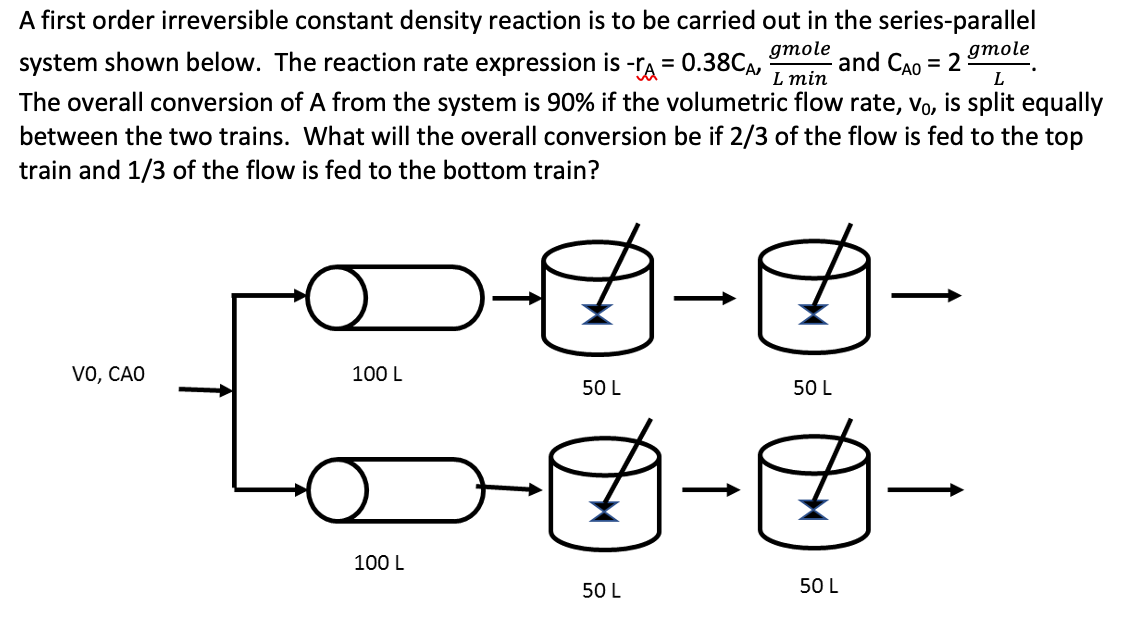
A first order irreversible constant density reaction is to be carried out in the series-parallel system shown below. The reaction rate expression is rA=0.38CA,Lmingmole and CA0=2Lgmole. The overall conversion of A from the system is 90% if the volumetric flow rate, v0, is split equally between the two trains. What will the overall conversion be if 2/3 of the flow is fed to the top train and 1/3 of the flow is fed to the bottom train
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


