Question: A first-order reaction requires 72 seconds for the reactant concentration to drop to 25% of the initial value. What would be the half-life of this
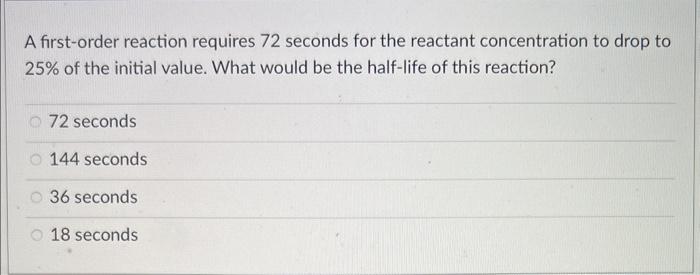
A first-order reaction requires 72 seconds for the reactant concentration to drop to 25% of the initial value. What would be the half-life of this reaction? 72 seconds 144 seconds 36 seconds 18 seconds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


