Question: The integrated rate law allows chemists to predict the reactant concentration after a certain amount of time, or the time it would take for a

![a first-order reaction is: [A]=[A]0ekt Now say we are particularly interested in](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8ee43daba9_95566f8ee437e40a.jpg)
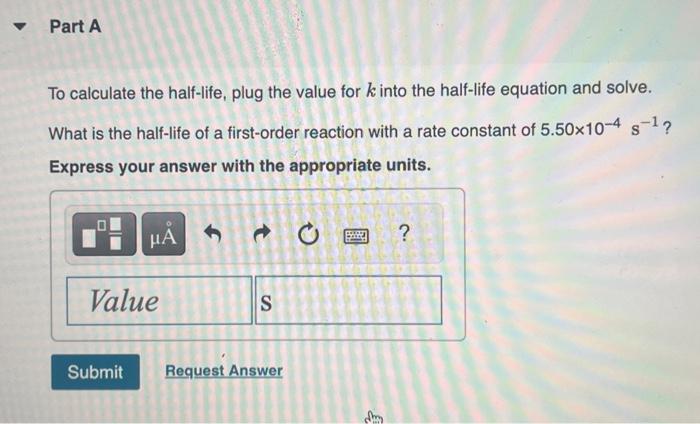
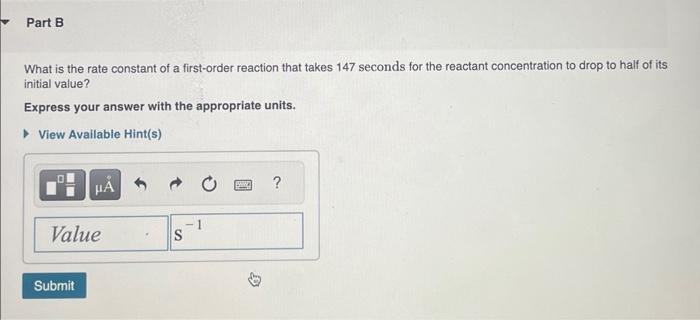
The integrated rate law allows chemists to predict the reactant concentration after a certain amount of time, or the time it would take for a certain concentration to be reached. The integrated rate law for a first-order reaction is: [A]=[A]0ekt Now say we are particularly interested in the time it would take for the concentration to become onehalf of its initial value. Then we could substitute 2[A]0 for [A] and rearrange the equation to: t1/2=k0.693 This equation calculates the time required for the reactant concentration to drop to half its initial value. In other words, it calculates the half-life. To calculate the half-life, plug the value for k into the half-life equation and solve. What is the half-life of a first-order reaction with a rate constant of 5.50104s1 ? Express your answer with the appropriate units. What is the rate constant of a first-order reaction that takes 147 seconds for the reactant concentration to drop to half of its initial value? Express your answer with the appropriate units. A certain first-order reaction has a rate constant of 3.20103s1. How long will it take for the reactant concentration to drop to 81 of its initial value? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


