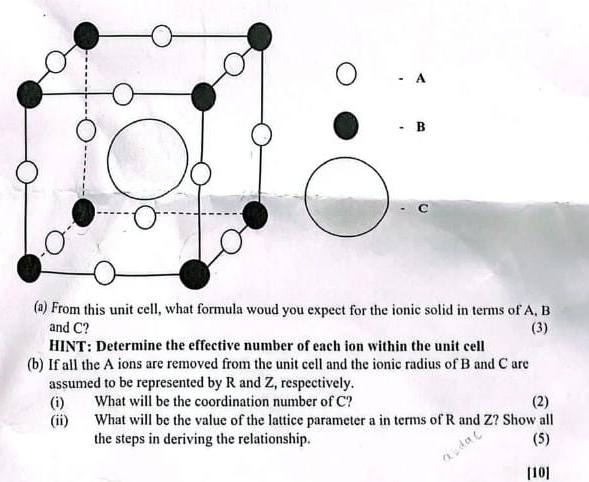
Question: ( a ) From this unit cell, what formula woud you expect for the ionic solid in terms of A , B and C ?
a From this unit cell, what formula woud you expect for the ionic solid in terms of A B and
HINT: Determine the effective number of each ion within the unit cell
b If all the A ions are removed from the unit cell and the ionic radius of and are assumed to be represented by and respectively.
i What will be the coordination number of
ii What will be the value of the lattice parameter a in terms of and Show all the steps in deriving the relationship.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


