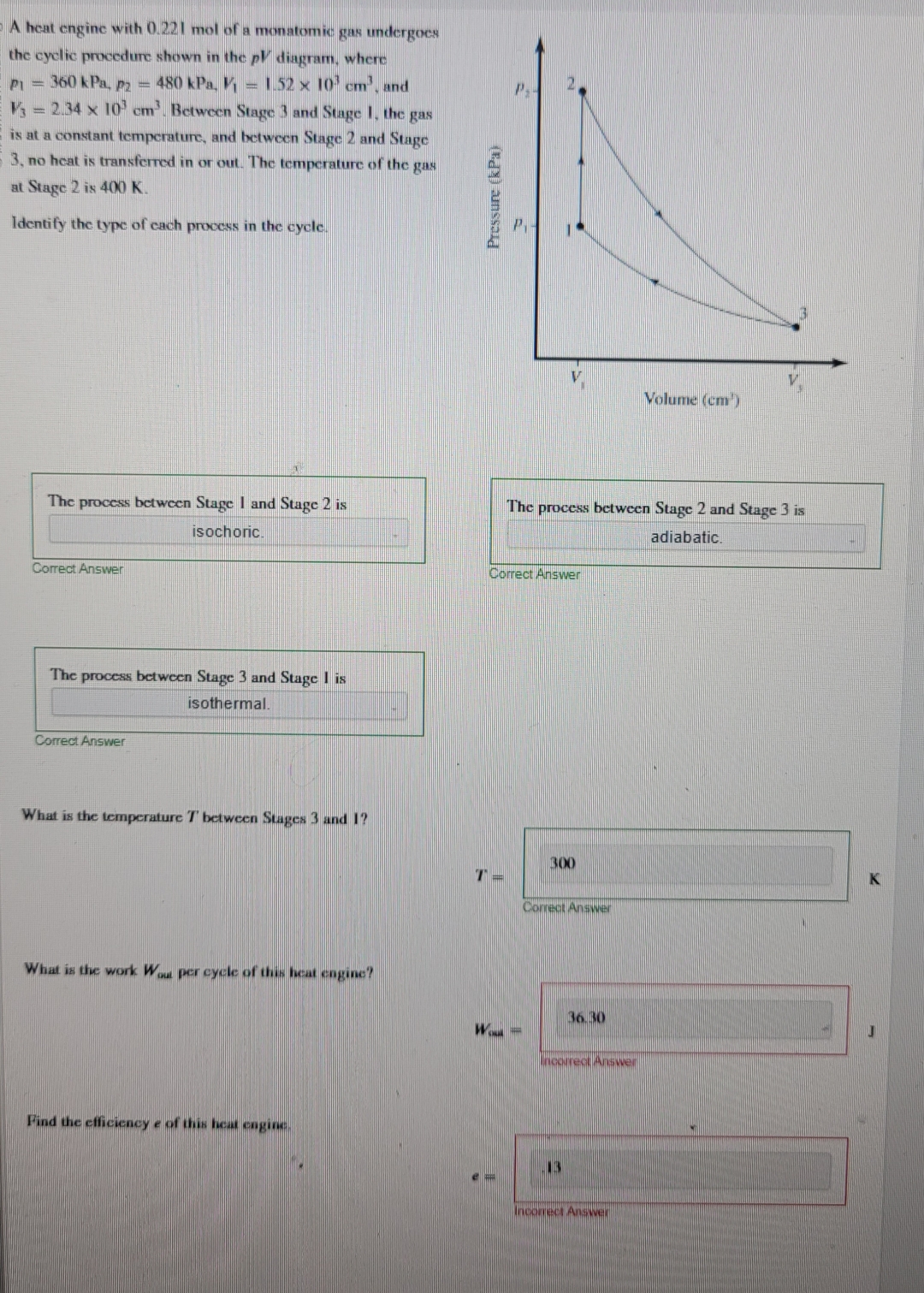
Question: A heat engine with 0 . 2 2 1 mol of a monatomic gas undergoes the cyclic procedure shown in the p V diagram, where
A heat engine with mol of a monatomic gas undergoes the cyclic procedure shown in the diagram, where kPa,kPa, and Between Stage and Stage the gas is at a constant temperature, and between Stage and Stage no heat is transferred in or out. The temperature of the gas at Stage is K
Identify the type of each process in the cycle.
The process between Stage and Stage is
isochoric.
Correct Answer
Correct Answer
What is the temperature between Stages and
What is the work per cycle of this heat engine?
Find the efficiency e of this heat cagine.
The process between Stage and Stage is adiabatic.
Correct Answer
Cornect Answer
Whewreat Aniswer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


