Question: A. Is the reaction first order or second order with respect to [C12H22O11]? B. What is the rate constant? C. Using this rate constant, calculate
A. Is the reaction first order or second order with respect to [C12H22O11]?
B. What is the rate constant?
C. Using this rate constant, calculate the concentration of sucrose at 39, 80, 140, and 210 min if the initial sucrose concentration was 0.316 M and the reaction was first order in sucrose. Express your answers using two significant figures separated by commas.
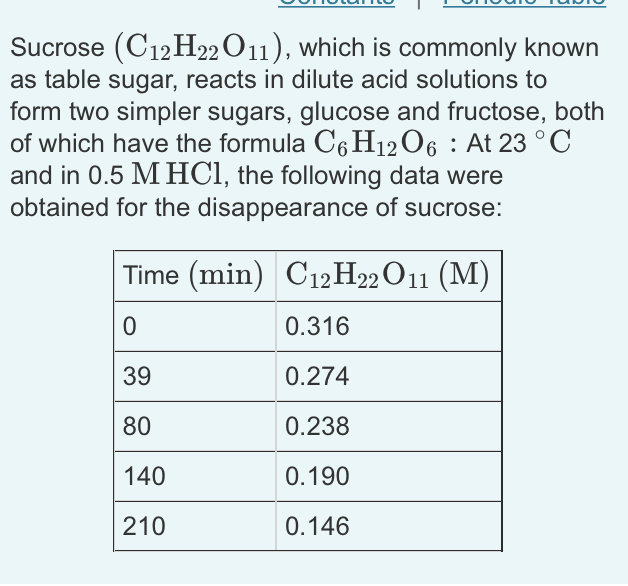
Sucrose (C12H22 O11), which is commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6 : At 23 C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) C12H22 O11 (M) 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


