Question: A liquid - phase reaction takes place in a CSTR of volume V R in the presence of a large excess of reactant B .
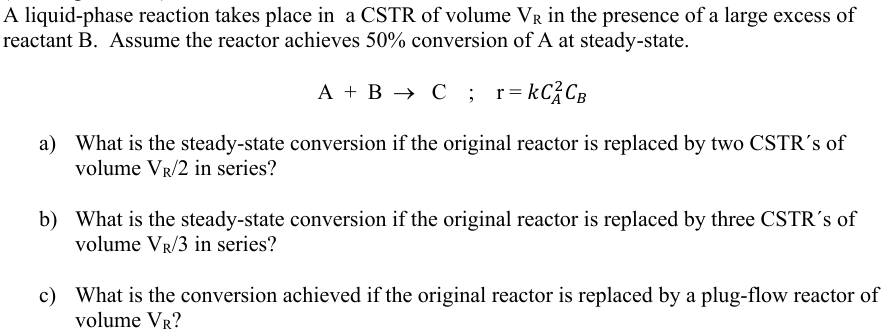
A liquidphase reaction takes place in a CSTR of volume in the presence of a large excess of reactant B Assume the reactor achieves conversion of A at steadystate.
;
a What is the steadystate conversion if the original reactor is replaced by two CSTRs of volume in series?
b What is the steadystate conversion if the original reactor is replaced by three CSTRs of volume in series?
c What is the conversion achieved if the original reactor is replaced by a plugflow reactor of volume
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


