Question: A monatomic gas is slowly and adiabatically compressed from initial conditions T 0 and P 0 to twice the original pressure. The equation of state
A monatomic gas is slowly and adiabatically compressed from initial conditions T and P to twice the original pressure. The equation of state of this gas is P kB Tv b where v is the volume per particle and b is a constant. Note that this is not an ideal gas and therefore you should not make any assumptions as such. What is the final temperature of the gas? How much work per molecule is required in order to accomplish this change of state?
Answer and Tip Show all the steps for this solution
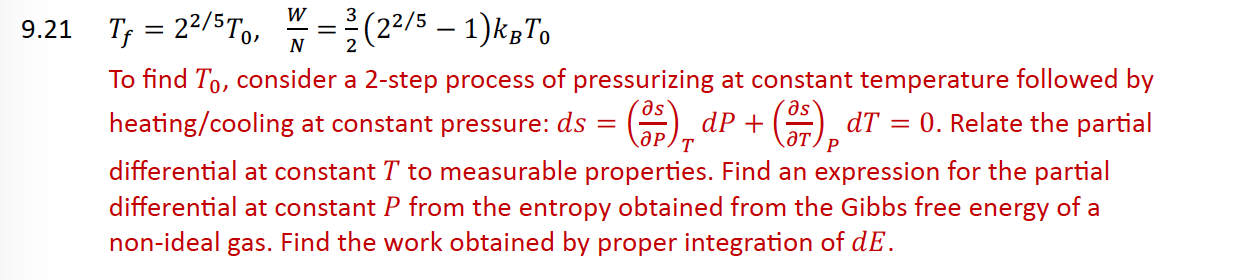
To find consider a step process of pressurizing at constant temperature followed by
heatingcooling at constant pressure: Relate the partial
differential at constant to measurable properties. Find an expression for the partial
differential at constant from the entropy obtained from the Gibbs free energy of a
nonideal gas. Find the work obtained by proper integration of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


